the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Physical protection of soil carbon stocks under regenerative agriculture
Sam G. Keenor
Rebekah Lee
Brian J. Reid
Regenerative agriculture is emerging as a strategy for carbon sequestration and climate change mitigation. However, for sequestration efforts to be successful, long-term stabilisation of Soil Organic Carbon (SOC) is needed. This can be achieved either through uplift in recalcitrant carbon stocks, and/or through physical protection and occlusion of carbon within stable soil aggregates. In this research soils from blackcurrant fields under regenerative management (0 to 7 years) were assessed. Soils from under the blackcurrant bush crop (bush (ca. 40 % of the field area)), and the alleyways between the blackcurrant crop rows (alley (ca. 60 % of the field area) were considered. Soil bulk density (SBD), soil aggregate fractions (proportions of water stable aggregates vs. non-water stable aggregates (WSA and NWSA)), soil carbon content, and carbon stability (thermally recalcitrant carbon vs. thermally labile carbon) were assessed. From this, long term carbon sequestration potential was calculated from both recalcitrant and occluded carbon stocks (both defined as stabilised carbon). Results indicated favourable shifts in the percentage of NWSA:WSA with time, increasing from 27.6 %:5.8 % (control arable field soil) to 12.6 %:16.0 % (alley soils), and 16.1 %:14.4 % (bush soils) after 7 years. While no significant (p≥0.05)) changes in whole field (area weighted average of alley and bush soils), recalcitrant carbon stocks were observed after 7 years, labile carbon stocks increased significantly (p≤0.05) from 10.44 to 13.87 t C ha−1. Furthermore, as a result of the occlusion of labile carbon within the WSA fraction, total stabilised carbon increased by 1.7 t C ha−1 over the 7 year period. This research provides valuable insights into the potential for carbon stabilisation and long-term stability prognoses in soils managed under regenerative agriculture practices, highlighting the important role which soil aggregate stability plays in the physical protection of carbon, and potential therein to deliver long-term carbon sequestration.
- Article
(1709 KB) - Full-text XML
-
Supplement
(485 KB) - BibTeX
- EndNote
Land use change, conventional land management practice, and aggressive agricultural techniques remain key drivers of soil damage and degradation (Lal, 2001; Lambin et al., 2001; Foley et al., 2005; Pearson, 2007; Smith, 2008; Al-Kaisi and Lal, 2020). Without a shift to more sustainable approaches future agricultural productivity will be endangered, and with it the loss of food and economic security for many around the world (Zika and Erb, 2009; Tilman et al., 2011; Sundström et al., 2014).
The effects of soil degradation can greatly reduce environmental and ecosystem quality and function (Dominati et al., 2010; Power, 2010; Lal, 2015; Montanarella et al., 2016; Sanderman et al., 2017; IPBES, 2018). At landscape scales, soil degradation compounds and threatens desertification and biodiversity loss (Zika and Erb, 2009; Power, 2010; Orgiazzi and Panagos, 2018; Huang et al., 2020), while making significant contributions to greenhouse gas emissions and climate change (Lal, 2004; Smith et al., 2020). Globally, agriculture is associated with roughly a third of total land use and nearly a quarter of all global greenhouse gas emissions each year (Foley et al., 2011; Smith et al., 2014; Newton et al., 2020). To date it is estimated that more than 176 Gt of soil carbon has been lost to the atmosphere (IPBES, 2018), with approximately 70 %–80 % of this (∼ 130–140 Gt) as a direct consequence of anthropogenic land management and soil cultivation (Sanderman et al., 2017; Lal et al., 2018; Smith et al., 2020). Meanwhile the area of land affected by desertification globally has been reported to exceed 25 % and is expanding each year (Huang et al., 2020).
A key mechanistic step in the wider degradation of soil and soil carbon loss, is through the loss and destruction of stable soil aggregates and associated SOC mediated by conventional agricultural practice and soil disturbance (Smith, 2008; Baveye et al., 2020).
Soil aggregates that remain stable and resist disaggregation when exposed to water (water stable aggregates) are key determinants of soil structure and stability (Whalen et al., 2003), and act as an important indicator of overall soil quality due to their influence on wider soil properties (Lehmann et al., 2020; Rieke et al., 2022). Soil aggregate formation, as facilitated by SOC, assists the stabilisation and storage (through occlusion and physical protection) of carbon and imparts resilience to soils against erosion and climate change while providing hydrological benefits and enhancing soil fertility (Lal, 1997; Abiven et al., 2009; Kasper et al., 2009; Chaplot and Cooper, 2015; Veenstra et al., 2021; Rieke et al., 2022).
The formation and persistence of stable soil aggregates is instrumental in soil carbon sequestration (Lal, 1997; Six et al., 1998; Abiven et al., 2009), in particular due to the physical protection of labile carbon within soil aggregates which minimise biogenic and oxidative decay of SOC (Brodowski et al., 2006; Smith, 2008; Schmidt et al., 2011; Berhe and Kleber, 2013). Soil aggregates can be classified by their formation conditions; biogenic (decomposition of organic matter and action of soil fauna), physicogenic (soil physical and chemical processes) and intermediate (a combination of both factors) (Ferreira et al., 2020). Additionally, land management practice can influence these formation conditions and the stability or destruction of soil aggregates (Lal, 1997; Mikha et al., 2021).
It is important, when considering carbon sequestration that we acknowledge not all carbon is equal, with long-term resistance to degradation being conferred through; (i) inherent recalcitrance of the carbon, and (ii) physical protection of the carbon (occlusion within soil aggregates). Thus, when considering soil carbon sequestration as a solution to climate change it is imperative that we differentiate between carbon which is transient and carbon which endures.
By adopting more sustainable management practices, agriculture can transition from a negative to a positive force for the environment; providing and enhancing a variety of key ecosystem services (water regulation, soil properties regulation, carbon sequestration and biodiversity support) (de Groot et al., 2002; Dominati et al., 2010; Power, 2010; Baveye et al., 2016; Keenor et al., 2021). Herein, regenerative agriculture offers opportunities to produce food and other agricultural products with minimal negative, or even net positive outcomes for society and the environment; potentially improving farm profitability, increasing food security and resilience, and mitigating climate change (Al-Kaisi and Lal, 2020; Newton et al., 2020).
Regenerative agriculture practice may include the key concepts of: (i) reducing/limiting soil disturbance; (ii) continuous soil cover (perennial crops/vegetation, litter or mulch), (iii) increased use of organic matter inputs; (iv) maximised crop nutrient and water-use efficiency; (v) integrating livestock; (vi) reducing or eliminating synthetic inputs (fertilisers and pesticides etc.); and (vii) increasing and broadening stakeholder engagement and employment (Newton et al., 2020; Paustian et al., 2020; Giller et al., 2021).
Adoption of regenerative practices such as no/minimum-till techniques increases the extent of soil aggregation and improves long-term carbon storage potential (Lal, 1997; Gál et al., 2007; Ogle et al., 2012; Lehmann et al., 2020). Furthermore, in addition to providing physical protection to more labile forms of soil carbon, the improved soil aggregation enhances resilience to the effects of drought and erosion, and provides better hydrological function and structure to the soil (Abiven et al., 2009; Bhogal et al., 2009; Baveye et al., 2020; Ferreira et al., 2020; Martin and Sprunger, 2022). No/minimum till techniques have been adopted worldwide and in a variety of agricultural contexts to help reduce soil erosion and SOC mineralisation, increase crop yields and minimise input costs all while building soil organic matter (Sisti et al., 2004; Pittelkow et al., 2015; Ferreira et al., 2020; Kan et al., 2021). Adoption of no/minimum till, coupled with the incorporation of perennial crops has been reported to significantly increase SOC content within the top 30 cm of a soil when compared with conventional tillage and yearly biomass harvest and removal (Gál et al., 2007; Ogle et al., 2012; Ledo et al., 2020).
Conversion of agricultural land from conventional to regenerative management may increase macro-aggregation and aggregate stability (Lal, 1997), and by extension, provide the means to protect labile soil carbon; thus, enhancing long-term soil carbon sequestration efforts (Six et al., 1998; Brodowski et al., 2006; Smith, 2008; Schmidt et al., 2011; Berhe and Kleber, 2013). Furthermore, adoption of regenerative methods can also lessen machinery costs, working hours and direct carbon emission (Kasper et al., 2009). Indeed, resulting from the adoption of no-till methods, it is estimated that global emission reductions of approximately 241 Tg CO2e have been achieved since the 1970s (Al-Kaisi and Lal, 2020).
To evaluate the influence of transitioning from conventional agricultural management to regenerative soft fruit production, a field experiment was undertaken on a commercial blackcurrant farm in Norfolk, UK. The experiment evaluated 5 blackcurrant fields managed regeneratively for increasing lengths of time (0–7 years of establishment), and contrasted against a conventionally managed arable field, evaluated as a datum. The research assessed carbon stocks across the regimes and thereafter the proportion of carbon stocks associated with the water stable aggregate (WSA) and non-water stable aggregate (NWSA) soil fractions, with respect to the soil under the blackcurrant bush crop (bush soil) and in between the rows of the blackcurrant crop (alley soils), and at field scale (alley and bush soils collectively). Thermogravimetric Analysis (TGA) was used to differentiate thermally labile and thermally recalcitrant carbon pools, and their association to the respective soil fractions, a proxy for wider carbon stability (Plante et al., 2005, 2011; Gregorich et al., 2015; Nie et al., 2018; Mao et al., 2022). The research sought to test the hypothesis that a switch from a high soil disturbance conventional arable system to a no soil disturbance regenerative perennial soft fruit production system, would increase total soil carbon stock with time and that this carbon stock would become increasingly stabilised. With this increased carbon stored either as occluded carbon (held within WSA, conferring physical protection to these stocks), and/or with greater inherent resistance to degradation (i.e. thermally recalcitrant carbon). A glossary of terms defining different soil carbon pools and soil fractions considered in this research is provided in Table S1 in the Supplement.
2.1 Field Experiment
This research was undertaken at Gorgate Farm, Norfolk, UK (52°41′58′′ N 0°54′01′′ E). The farm is part of the wider Wendling Beck Nature Recovery Project, a regenerative farming and environmental landscape management program set in approximately 750 ha. The field experiment comprised 5 blackcurrant fields established on sandy-loam soils in 2019, 2017, 2015, and 2013 (these representing 1, 3, 5, and 7 years since soil disturbance, respectively) and a conventionally managed arable field drilled with winter wheat as a datum (0 years since soil disturbance). Soil samples were collected in late June 2021, immediately prior to the harvest of the blackcurrant crops and a month prior to harvest of the winter wheat crop. Field cropping history in both the blackcurrant and the arable regimes (2014–2021) is shown in Fig. 1.
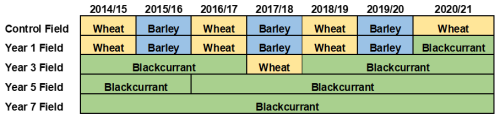
Figure 1Field cropping history for the arable control and regenerative blackcurrant fields (2014–2021). Discrete boxes represent one full cropping cycle and where applicable re-planting of new bushes.
The regeneratively managed blackcurrant fields were planted using a conservation strip tillage approach: bushes are planted as field length strips, leaving alleyways approximately 2 m wide. Blackcurrant bushes occupied approximately 40 % of the field and the alleyways between the crops approximately 60 %. Once planted, the blackcurrant crop required minimal intervention beyond the yearly harvest, pruning, fertilisation and sowing of cover crops in the alleyways. Soil remained covered year-round between the blackcurrant crop, with a diverse grazing cover crop through the autumn and winter months, and a summer fallow covering crop during the spring and summer months, both directly drilled (Table S2). Furthermore, the blackcurrant crop was treated with bi-yearly sprays of compost tea and urea fertiliser (78 and 100 kg ha−1 pre and post flowering of the currants, respectively). Plant trimmings and residue were left on the soil surface in situ following yearly pruning and seasonal leaf drop. Comparatively the control comprised a conventionally managed arable field adjacent to the blackcurrant fields and occupying the same sandy-loam soil type. This field was cultivated yearly to 30 cm depth and had been drilled with winter wheat in a wheat/barley rotation for the preceding 6 years. Furthermore, during cultivation the previous crop stubble was re-incorporated, and the crop was subsequently treated with a urea-based fertiliser at a rate of 100 kg ha−1 post emergence. In the case of blackcurrants being replanted into previously used fields (e.g., the year 5 field, Fig. 1), these soils were no longer classified as under continuous regenerative management. The removal of previously established bushes and the subsequent soil disturbance caused through cultivation, and replanting constituted a clear disruption to ongoing regenerative practice, and goal of no soil disturbance. As such, this site was more accurately characterised by the initiation of a new cycle of regenerative management, reflecting this transition and the accompanied soil disturbance, rather than as a continuation of the previous management.
2.2 Soil Sampling
Soil core samples (0–7.5 cm; n=5) were collected from beneath the blackcurrant bushes and at the centre of the alleyways of each blackcurrant field using a Dent soil corer. Whilst we acknowledge that collection and measurement of samples to a greater depth would be advantageous for determination of potential changes in soil composition of different strata, given the physiological differences between these different soil layers (Penman et al., 2003; Rovira et al., 2022), in practice the investigation was subject to limitations to our sampling protocol. As a result, the discussion of soil compositional characteristics and changes is kept relative to the topsoil (7.5 cm soil depth). Further soil core samples (n= 5) were randomly collected from a conventionally managed arable field. Soil samples were sealed and retained in cold storage (≤ 4 °C) prior to laboratory analysis. Soil cores were subsequently oven dried (40 °C for 24 h) and soil bulk density calculated from the dry sieved (2 mm) bulk soil prior to soil fractionation (n=5).
2.3 Soil Fractionation
Soil fractionations, namely, Water Stable Aggregates (WSA), Non-Water Stable Aggregates (NWSA) and sand (Table S1), were established using a capillary-wetting wet sieving method, adapted from Seybold and Herrick (2001). To generate these different soil fractions, the previously dried bulk density samples (n= 5) were dry sieved (2 mm) to remove all debris and material ≥ 2 mm, yielding the bulk soil fraction. Subsequently, this 2 mm sieved bulk soil (100 g) was placed on 63 µm sieves and slowly wetted with de-ionised water. Once damp, samples were submerged and oscillated under de-ionised water (manually agitated at 30 oscillations per minute in 1.5 cm of water for 5 min). Material that passed through the 63 µm sieve was collected and dried (40 °C for 24 h) and then weighed, yielding the NWSA fraction. The soil retained on the 63 µm sieve was further processed using sodium hexametaphosphate (HMP) solution (0.02 M), to disaggregate any water stable aggregates from the remaining material and separate these from the sand and inorganic material present in the sample. The material remaining on the 63 µm sieve was then dried (40 °C for 24 h); and designated as the sand fraction (the overall change in sand fraction has been discounted to focus reporting on NWSA or WSA fractions). The WSA fraction (That which passed through the 63 µm sieve after receiving the HMP treatment) was subsequently established by calculation (Eq. 1):
2.4 Total Carbon Content by Elemental Analysis
Dry bulk soil, and the separated soil fractions (sand and NWSA), were milled to produce a fine powder, and subsequently samples (20 mg; n= 4) packed in 8 × 5 mm tin capsules. An elemental analyser (Exeter CHNS analyser (CE440)) was used to determine elemental abundance of C. Instruments were pre-treated within conditioning samples (acetanilide 1900 µg), a blank sample (empty capsule) and an organic blank sample (benzoic acid 1700 µg) prior to analysis, and standard reference materials (acetanilide 1500 µg) were run alongside samples (every 6th run) for QA/QC (a precision threshold of ±1 SD of the mean from the standard reference material) (Hemming, 2024). WSA fraction carbon content was subsequently established by calculation (variation of Eq. 1).
2.5 Thermogravimetric Assessment of SOC Stability
Thermal stability of the SOC in the bulk soil, and the separated soil fractions (sand fraction and NWSA fraction) were assessed using a thermo-gravimetric analyser (Mettler Toledo TGA/DSC 1). Samples (n= 2) were contained in 70 µL platinum crucibles. Samples were heated, in an inert atmosphere, at a rate of 10 °C min−1 from 25 to 1000 °C. TGA data was subsequently used to ascribe the thermally labile and thermally recalcitrant carbon contents (hereafter referred to as just labile/recalcitrant, respectively) of the bulk soil and soil fractions, as well as any inorganic carbon within the samples. Data was split into 4 distinct phases by temperature range according to organic matter attrition windows as stated in Mao et al. (2022): (i) 25–125 °C (moisture evaporation), (ii) 125–375 °C (labile components) and, (iii) 375–700 °C (recalcitrant components), (iv) 700–1000 °C (inorganic components). WSA fraction carbon stabilities were subsequently established by calculation (variation of Eq. 1).
2.6 Carbon Assessment
Soil carbon was assessed as total SOC, soil fraction C (NWSA associated carbon, and WSA associated carbon respectively), total labile and recalcitrant C, occluded carbon (physically protected) and unstabilised C (Table S1). These fractions were not defined relative to particulate organic matter (POM) or mineral associated organic matter (MAOM), due to the method of sample preparation being unsuitable to accurately prescribe these fractions (Lavallee et al., 2020). In addition, C was further assessed on a total field stock basis (in t C ha−1). To calculate the C content of both the alley and bush soils (or the sum of their relative fractions) was multiplied by the relevant soil bulk density measure and the depth of sampling (ca. 7.5 cm) and subsequently added together with acknowledgment of their proportion of the field (60 % alley and 40 % bush, respectively), as set out in (Eq. 2):
2.7 Statistical Analysis
Significant differences between the field sites were determined using post hoc tests on one-way ANOVA with Tukey's HSD, and data significance set to 95 % (p≤ 0.05) (ANOVA; IBM SPSS 28). Significant differences between the individual regimes within field sites (alley soil vs. bush soil) were determined using two tailed t-tests, with data significance set at two levels of confidence; 95 % (p≤ 0.05), and 99 % (p≤ 0.01) (independent samples t-test; IBM SPSS 28).
3.1 Bulk Density
When considering soil stability, soil bulk density (SBD) provides significant insights into the arrangement and structure of soil particles, and the extent of soil aggregation (Al-Shammary et al., 2018). As SBD accounts for the total volume that soils occupy (including the mineral, organic and pore space components), it is a key indicator of soil condition (Chaudhari et al., 2013; Allen et al., 2011). Furthermore, SBD maintains a close correlation to concentrations of organic matter and carbon within the soil, where soils become depleted in carbon, SBD tends to increase, potentially leading to compaction of soil structures (Allen et al., 2011).
SBD was observed to decrease significantly (p≤ 0.05) in both the alley and bush soils of all regeneratively managed fields relative to the conventional control (1.75 g cm−3) (Fig. 2). In the alley soils, SBD was observed to decrease successively with each additional year under regenerative management; from 1.35 g cm−3 in the year 1 soil, to 1.15 g cm−3 in the year 7 soil, with no significant differences observed between the regeneratively managed fields (p≥ 0.05). While in the bush soils, SBD decreases were not successive between the regeneratively managed fields (Fig. 2). Intra-regenerative field comparison showed the greatest decrease in bush soil SBD (significant (p≤ 0.05)) was observed between the year 1 and year 3 fields, reducing from 1.32 g cm−3 in to 1.07 g cm−3, before increasing (not significantly (p≥ 0.05)) in the year 5 and 7 fields (to 1.18 and 1.16 g cm3 respectively) (Fig. 2), likely a consequence of increased stoniness (thus reducing core mass and volume) in the year 3 samples (Table S3). When compared pairwise, SBD in the alley and the bush soils of the regeneratively managed fields were observed to be broadly similar, with only one pair (year 3) measuring a significant difference (p≤ 0.05) of 1.27 and 1.07 g cm−3 respectively (Fig. 2). However, this difference likely related more to the underlying soil physiology and stoniness of the samples collected than to differences in management practice (Fig. S3).
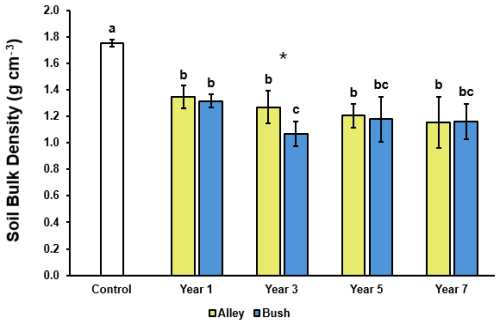
Figure 2Soil bulk density (n= 5) of alley (yellow) and bush (blue) regimes with increasing years of establishment. Error bars represent ±1 SD. For a given regime (alley or bush) dissimilar lower-case letters indicate significant (p≤ 0.05) differences across the timeseries. At a given timepoint, * indicates a significant difference (p<0.05) between the alley and bush regimes.
None of the soils measured in this investigation were observed to exceed the root limiting soil density factor of 1.8 g cm−3 in sandy soil types, suggesting no significant detriment to plants growth from soil compaction (Kaufmann et al., 2010; Shaheb et al., 2021). Furthermore, the overall trend of soil bulk density reduction seen over the course of the 7-year period (Fig. 2) is likely a consequence of both increased aggregate stability and quantity of stable aggregates (Sect. 3.2) alongside increases in soil carbon stocks (Sect. 3.3) (Topa et al., 2021; Rieke et al., 2022; Kasper et al., 2009).
3.2 Soil Fractionation
Proportions of WSA and NWSA in both the alley and bush soils were seen to change significantly (p≤ 0.05) with increased time under regenerative management (Fig. 3). NWSA in both the regimes reduced in fractional share significantly (p≤ 0.05) over the 7 years of establishment, while the WSA fractional share increased significantly over time (p≤ 0.05) (Fig. 3; Table S3). Such changes were likely due to halting of soil tillage (with a decrease in NWSA, and commensurate increase in WSA evident in the first year of no-till adoption) and further enrichment with increasing time since soil disturbance. Furthermore, these shifts in NWSA vs WSA proportions were noted to be commensurate with soil carbon increases (Sect. 3.3) and SBD decreases (Sect. 3.1), Collectively these changes may suggest enhanced soil aggregate stability and cohesion (Abiven et al., 2009; Six et al., 2004; Kasper et al., 2009).
NWSA fractions in the alley soils decreased successively with time, from a total of 27.6 % in the control soil to 12.6 % in the year 7 soil, with significant reductions (p≤ 0.05) measured between the control soil and all regeneratively managed soils (Fig. 3; Table S3). Additionally, NWSA in the year 7 soil was measured to be significantly lower (p≤ 0.05) than all other regeneratively managed soils (Fig. 3; Table S3). In the bush soil, NWSA fractions were also observed to decrease significantly (p≤ 0.05) in all regeneratively managed soils relative to the control, reducing from 27.6 % in the control to 16.1 % in the year 7 soil (Fig. 3; Table S3). However, this decrease was not successive, with the greatest reduction measured in the year 1 soil (15.2 %), however, no significant differences (p≥ 0.05) were observed between any of the regeneratively managed soils. When compared pairwise significant differences (p≤ 0.01) between the alley and bush soil NWSA were observed in the year 5 and year 7 soils, significantly (P≤ 0.01) lower in the alley soils than the bushes (15.9 % vs. 18.8 % in year 5; 12.6 % vs. 16.1 % in year 7, respectively) (Fig. 3; Table S3).
Conversely WSA fractions in the alley soils increased broadly with age of establishment, from 5.8 % in the control soil to 16.0 % in the year 7 soil, with significant increases (p≤ 0.05) measured between the control soil (5.8%) and both the year 5 and year 7 soils (10.3 % and 16.0 % respectively), (Fig. 3; Table S3). Additionally, the WSA fraction in year 7 was observed to be significantly greater (p≤ 0.05) than in all other regeneratively managed soils (Fig. 3; Table S3). In the bush soils, the WSA fraction was also observed to generally increase with time, from 5.8 % in the control soil to 14.4 % in the year 7 soil; with significant increases (p≤ 0.05) measured in the year 5 and year 7 soils (11.0 % and 14.4 % respectively) (Fig. 3; Table S3). Within the regeneratively managed soils, significant differences (p≤ 0.05) were also observed between the year 5 soil and the year 3 soil, and between the year 7 soil and years 1 and 3 soils (Fig. 3; Table S3). When compared pairwise no significant differences (p≥ 0.05) were observed for the WSA content of the alley and bush soils in each year of regenerative management (Fig. 3; Table S3).
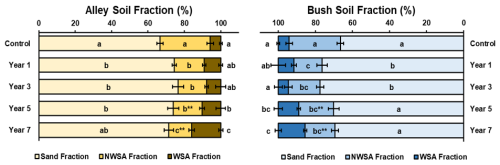
Figure 3Sand, Non-Water Stable Aggregates (NWSA), and Water-Stable Aggregates (WSA) fractions (% total mass)) (n= 5) of alley (left) and bush (right) regimes with increasing years of establishment. Error bars represent ±1 SD. For a given regime (alley or bush) dissimilar lower-case letters indicate significant (p≤ 0.05) differences across the timeseries. At a given timepoint, the * indicates a significant difference (p≤ 0.05) between the alley and bush regimes. indicates a significant difference (p≤ 0.01), between the alley and bush regimes.
3.3 Soil Carbon and Thermal Stability
SOC was observed to increase in both the alley and bush soils over time (Fig. S1), with significant increases (p≤ 0.05) in the year 5 bush soil (22.3 g kg−1 C) and both the alley and bush soils of year 7 (29.9 and 23.8 g kg−1 C respectively) relative to the control soil (16.6 g kg−1 C) (Fig. S1). Such increases in SOC likely pertaining to lower carbon turnover from reduced soil disturbance, and increased carbon input; from the perennial root systems of the blackcurrant bushes and alley conservation strip, and litter/residue derived from crop pruning and seasonal leaf fall, relative to the yearly removal of biomass in the conventionally managed field (Ledo et al., 2020; Kan et al., 2021). While increases in SOC were more pronounced in the alley soils than in the bush soils, no significant (p≥ 0.05) differences were observed when compared pairwise (Fig. S1).
The relative stability of soil carbon is an underlying feature of its inherent environmental value: biological function and soil biodiversity rely heavily upon easily degradable carbon pools with short residence times, while services such as carbon sequestration and long term storage rely upon the more stable recalcitrant carbon pools that can resist degradation (Dell'Abate et al., 2003; De Graaff et al., 2010; Kleber, 2010; Keenor et al., 2021; Martin and Sprunger, 2022). Thermal techniques such as thermogravimetric analysis can provide effective means of characterising these organic matter pools in the soil, defining the profile of SOC stability (Dell'Abate et al., 2000, 2003; Plante et al., 2005; Plante et al., 2011; Mao et al., 2022). Furthermore, this thermal stability can provide a proxy for biogenic decay and degradation of soil organic matter and carbon stocks (Plante et al., 2005; Plante et al., 2011; Gregorich et al., 2015; Nie et al., 2018; Mao et al., 2022).
Total labile and recalcitrant carbon pools were observed to increase broadly stepwise over the 7 year period relative to the control soil, with more labile carbon than recalcitrant carbon measured in both alley and bush soils in each field (Fig. 4). However, while increases in labile carbon were significant (p≤ 0.05) in both the alley and bush soils with time (years 5 and 7), no significant differences (p≥ 0.05) were observed in the recalcitrant carbon pool (Fig. 4).
Labile soil carbon measured in the alley soils increased in all regeneratively managed soils relative to the control soil. Significant increases (p≤ 0.05) were measured in both the year 5 and year 7 soils relative to the control (from 7.9 g kg−1 Clabile (control) to 13.6 and 17.6 g kg −1 Clabile, years 5 and 7 respectively) (Fig. 4). Additionally, significant differences (p≤ 0.05) in labile carbon were observed between the year 7 and years 1 and 3 alley soils (Fig. 4).
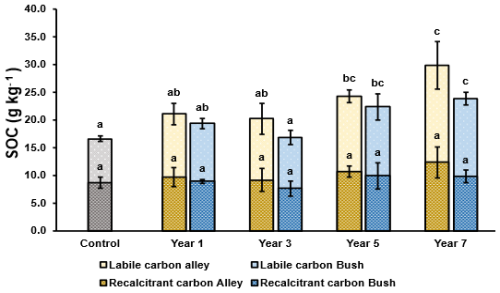
Figure 4Soil Organic Carbon (SOC) split by thermally recalcitrant (hashed) and thermally labile (plain) carbon pools (n= 5) in the alleyway (yellow) and bush (blue) regimes. Error bars represent ±1 SD. For a given regime (alley or bush) dissimilar lower-case letters indicate significant (p≤ 0.05) differences across the timeseries. At a given timepoint, * indicates a significant difference (p<0.05) between the alley and bush regimes.
In the bush soils, significantly greater (p≤ 0.05) labile carbon stocks were also measured in the year 5 and year 7 soils relative to the control (increasing from 7.9 to 12.4 and 13.9 g kg−1 C labile, respectively) (Fig. 4). Furthermore, significant differences (p≤ 0.05) were measured between regeneratively managed soils (year 5 and 7 vs. year 3; and year 7 vs. year 1) (Fig. 4). When compared pairwise, labile carbon in the alley soil increased by a total of 9.7 g kg−1 C labile, vs. Increase of 4.0 g kg−1 Clabile in the bush soil after 7 years of regenerative management, suggesting enhanced labile carbon stock growth in alley soils relative to bush soils (Fig. 4). However, no significant differences (p > 0.05) were observed between alley and bush soils of the same field (Fig. 4).
Recalcitrant carbon measured in the alley soils increased broadly stepwise relative to the control with time, from 8.7 g kg−1 Crecalcitrant (control) to 12.3 g kg−1 Crecalcitrant (year 7 soils), however none of these increases were significant (p≥ 0.05) (Fig. 4). In the bush soils, recalcitrant carbon was also observed to generally increase with time (not significantly (p≥ 0.05)). These increases were smaller than those observed within the alley soils, increasing (not significantly (p≥ 0.05)) from 8.7 g kg−1 C recalcitrant (control) to 9.9 g kg−1 C recalcitrant in the year 7 soil (Fig. 4). When compared pairwise, recalcitrant carbon stocks in the alley soil increased by a total of 3.6 g kg−1 C recalcitrant, compared with 1.2 g kg−1 C recalcitrant in the bush soil after 7 years of regenerative management, again suggesting increased carbon stock growth in the alley soils relative to the bush soils (Fig. 4). However, no significant differences (p>0.05) were observed between alley and bush soils of the same field (Fig. 4).
Considering both labile and recalcitrant carbon collectively, by year 7, the alley soil was observed to contain a total carbon content of 29.9 g kg−1 C (split as 17.6 g kg−1 Clabile and 12.3 g kg−1 Crecalcitrant), while the bush soil contained a total carbon content of 23.8 g kg−1 C (split as 13.9 g kg−1 C labile and 9.9 g kg−1 Crecalcitrant). In contrast, total carbon content in the control soil was 16.6 g kg−1 C (split as 7.9 g kg−1 Clabile and 8.7 g kg−1 Crecalcitrant) (Fig. 4).
3.4 Carbon Thermal Stability in Aggregate Fractions
Total labile and recalcitrant carbon pools, when split by soil fraction, were found to diverge over the 7 year period, with greater proportions of carbon (both labile and recalcitrant) observed in the WSA fraction while diminishing in the NWSA fraction with time. It is highlighted that despite their smaller fractional share (Sect. 3.2), WSA were substantially enriched in carbon relative to the NWSA fraction.
Labile carbon in the alley soils was observed to shift toward dominance of the WSA fraction with time, with significant decrease (p≤ 0.05) in the NWSA fraction and a non-significant increase (p≥ 0.05) in the WSA fraction over the 7 year period (Fig. 5A). Alley NWSA fraction labile carbon significantly decreased (p≤ 0.05) from 33.7 % in the control to 17.5 % in the year 7 soil. However, no significant differences (p≥ 0.05) were measured between the control and the other regeneratively managed soils (Fig. 5A). While alley WSA fraction labile carbon increased (not significantly (p≥ 0.05)) from 45.5 % in the control to 61.3 % in the year 7 soil (Fig. 5A). additionally, initial reductions in labile carbon were observed in year 1 and year 3 relative to the control (reducing to a low of 38.1 % in the year 3 soil), before rebounding in years 5 and 7. However no significant differences (p≥ 0.05) were observed between any of the regeneratively managed alley soils (Fig. 5A).
Labile carbon in the bush soils was similarly observed to shift toward dominance of the WSA fraction with time under regenerative management, culminating in reduced NWSA and increased WSA associated labile carbon by year 7. However, this trend was less pronounced than within the alley soil, with a significant difference (p≥ 0.05) only observed in the year 7 WSA fraction (Fig. 5B). Bush NWSA fraction labile carbon was observed to decrease (not significantly (p≥ 0.05)) between the control and year 7 soil, reducing from 33.7 % to 23.7 % respectively, additionally, no significant differences p≥ 0.05) were measured between the other regeneratively managed soils (Fig. 5B). While bush WSA fraction labile carbon increased (not significantly (p≥ 0.05)) between the control and year 7 soil (45.5 % to 54.8 %), however these changes were not as substantial as those observed in the alley soils (Fig. 5B). Additionally, while WSA associated labile carbon decreased in the year 3 soil to 28.2 %, (not significantly (p<0.05)), this was observed to rebound significantly (p≤ 0.05) from year 3 to year 7, showing overall increase in WSA associated labile carbon (Fig. 5B).When compared pairwise, a significant difference (p≤ 0.05) was observed between the NWSA fraction of year 5 alley and bush soils, with 23.7 % labile carbon in the alley soil relative to 33.8 % in the bush soil; no further significant differences (p≥ 0.05) were observed (Fig. 5A/B).
Recalcitrant carbon in the alley soils was also observed to enrich in WSA relative to the NWSA fractions over time, with the decrease in NWSA being significant (p≤ 0.05), while the increase in WSA was not significant (p≥ 0.05) over the 7 year period (Fig. 5C). Alley NWSA fraction recalcitrant carbon decreased broadly stepwise, with a significant decrease (p≤ 0.05) measured between the 7-year and control soil (from 33.2 % to 18.9 %) (Fig. 5C). Significant differences (p≤ 0.05) were also observed between the year 3 and year 7 soils, where NWSA fraction proportion increased to converge with the control in the year 3 soil (32.2 %), and thereafter decreasing in year 5 and year 7 (Fig. 5C). Alley WSA fraction recalcitrant carbon was observed to increase (not significantly (p≥ 0.05)) with time, from 50.1 % in the control to 64.5 % in the year 7 soil (Fig. 5C). Initial decreases in recalcitrant carbon were measured in the year 1 soil (not significantly (p≥ 0.05)) to 41.0 %), following thereafter subsequent stepwise increases in all other regeneratively managed soils (Fig. 5C). Recalcitrant carbon in the bush soils was also observed to increase the WSA fraction (not significantly (p≥ 0.05)) and decrease (not significantly (p≥ 0.05)) within the NWSA fraction with time, from the control soil to the year 7 soil (Fig. 5D). Bush NWSA fraction recalcitrant carbon was observed to decrease overall by year 7 (from 33.2 % in the control to 26.2 %), however, no significant differences (p≥ 0.05) were measured between any of the regeneratively managed soils and the control (Fig. 5D). Conversely, bush WSA fraction recalcitrant carbon was observed to increase overall from the control to year 7 (Fig. 5D). Initially (not significant (p≥ 0.05)) reductions were measured in year 1 and 3 relative to the control soil (decreasing from 50.1 % in the control to 36.4 % in the year 3 soil), before subsequently increasing stepwise to a total of 56.4 % in year 7 (however, this was not significantly different (p≥ 0.05) to the control) (Fig. 5D). When compared pairwise significant differences (p≤ 0.05) were observed between the NWSA fraction recalcitrant carbon stocks of both year 5 and year 7 soils, with 23.9 % and 18.9 % stored in the alley soils, vs. 34.1 % and 26.2 % stored in the bush soils respectively (Fig. 5C/D).
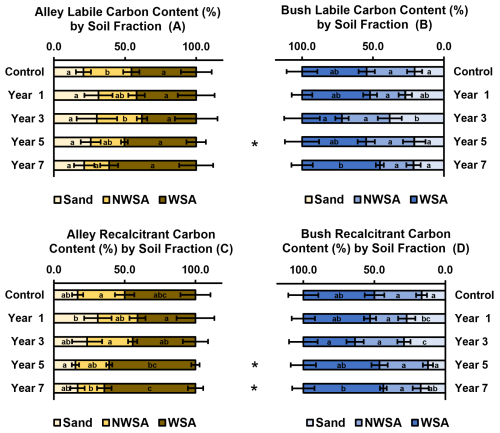
Figure 5Thermally labile (top) and thermally recalcitrant (bottom) SOC split by soil aggregate fraction (Sand, Non-Water Stable Aggregates (NWSA) and Water-Stable Aggregates (WSA)) as a total % of soil mass (n= 5), of alley (left) and bush (right) soils with increasing years of establishment. Error bars represent ±1 SD. For a given soil fraction (sand, NWSA, WSA) dissimilar lower-case letters indicate significant (p≤ 0.05) differences across the timeseries. At a given timepoint, the * indicates a significant difference (p≤ 0.05) between the alley and bush regimes. indicates a significant difference (p≤ 0.01), between the alley and bush regimes.
3.5 Aggregate Occlusion of Carbon
Despite the inherent degradability of the labile carbon stocks of in both NWSA and WSA aggregate structures, these can be considered as distinct carbon pools for the purpose of long-term carbon storage and stability (Six et al., 1998; McLauchlan and Hobbie, 2004). The ascribed occluded carbon pool considered the stabilised labile carbon stocks held within the WSA fraction (Sect. 3.4). Considered as such due to the long-term storage potential conferred by physical protection within the aggregate structures, and the physical separation of the carbon from its potential vectors of degradation – inhibiting the breakdown and decomposition of the carbon stored within (Six and Jastrow, 2002; McLauchlan and Hobbie, 2004; Smith, 2008; Gärdenäs et al., 2011; Plante et al., 2011; Dungait et al., 2012; Schrumpf et al., 2013). Conversely unstabilised carbon considered the labile carbon that contained within the NWSA fraction (Sect. 3.4), and thus with greater potential for degradation, due to the enhanced potential for carbon oxidation and decomposition by soil biota (Smith, 2008; Berhe and Kleber, 2013; De Gryze et al., 2006; Six et al., 1998; Dungait et al., 2012). Additionally, recalcitrant carbon (Sect. 3.3), was considered stabilised regardless of the soil aggregate pool in which it was contained (both WSA and NWSA) due to the relative stability of this carbon fraction.
Occluded carbon in the alley soils increased broadly stepwise with time, with increased occluded carbon content in all regeneratively managed soils relative to the control. However, this increase was only significant (p≤ 0.05) in the year 7 soil, increasing from 3.64 g kg−1 C in the control to 10.99 g kg−1 C over the 7 year period (Fig. 6). In the bush soil, occluded carbon was observed to follow a similar trend to that in the alley, increasing significantly (p≤ 0.05) from 3.64 g kg−1 C in the control to 7.66 g kg−1 in the year 7 soil (Fig. 6). However, a decrease (not significant (p≥ 0.05)) in the occluded carbon content of the year 3 bush soil was measured relative to the control soil, reducing to 2.64 g kg−1 C, before rebounding in years 5 and 7 (Fig. 6). When compared pairwise, no significant differences (p≥ 0.05) were observed between the occluded carbon contents of either the alley soils or bush soils, however a greater quantity of occluded carbon was measured within the alley soils relative to the bush soils in all but year 1 (Fig. 6).
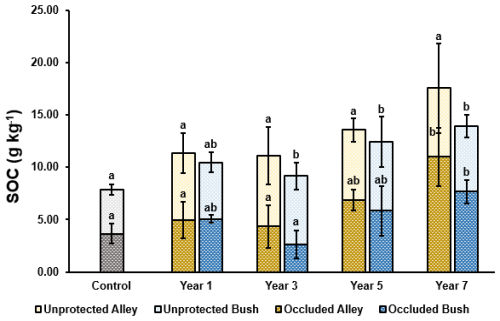
Figure 6Thermally labile SOC split by occluded (hashed) and unprotected (plain) carbon pools (n= 5) in the alley (yellow) and bush (blue) regimes. Error bars represent ±1 SD. For a given regime (alley or bush) dissimilar lower-case letters indicate significant (p≤ 0.05) differences across the timeseries. At a given timepoint, * indicates a significant difference (p<0.05) between the alley and bush regimes.
Unprotected carbon in the alley soils was observed to increase (not significantly (p≥ 0.05)) in all regeneratively managed soils relative to the control soil, ranging between 6.4 and 6.7 g kg−1 C, compared with 4.2 g kg−1 C in the control soil (Fig. 6). In the bush soil, unprotected carbon was observed to increase broadly stepwise, with significant increases (p≤ 0.05) in the years 3, 5 and 7 soils relative to the control, and increasing to a maximum of 6.6 g kg−1 (in the year 5 soil) relative to 4.2 g kg−1 C in the control soil (Fig. 6). When compared pairwise no significant differences (p≥ 0.05) were observed between the regeneratively managed soils, with unprotected carbon measuring similarly in both the alley soils and bush soils (Fig. 6).
3.6 Carbon Stability at Field Scale
Acknowledging proportions of alley and bush soils (60 % and 40 % of field area, respectively) and accommodating the influence of SBD (Sect. 3.1; Fig. 2), soil carbon contents (in g C kg−1) (Sect. 3.3; Fig. S1) were converted to carbon stocks (t C ha−1). These field scale soil carbon stocks were observed to increase (not significantly (p≥ 0.05)) by 1.74 t C ha−1 over the 7 year period relative to the control soil (from 21.98 to 23.72 t C ha−1) (Fig. S2).
When considering carbon stocks as split by labile and recalcitrant carbon pools, both were initially observed to decrease between the control and year 3 soil (Fig. 7A), likely in response to lower soil carbon inputs, arising from small infrequent litter drop of the young plants compared with the yearly incorporation of crop residues in the conventional system, and additionally soil disturbance during planting. The majority of this decrease occurred in the recalcitrant carbon stock, decreasing significantly (p≤ 0.05) from 11.54 to 7.62 t C ha−1, while labile carbon stock was observed to decrease gradually (not significantly (p≥ 0.05) from 10.44 to 9.22 t C ha−1 (Fig. 7A). Following this initial decrease, both labile and recalcitrant carbon stocks were observed to subsequently increase in years 5 and 7, by which point labile carbon stocks were observed to exceed those in the control (Fig. 7A).
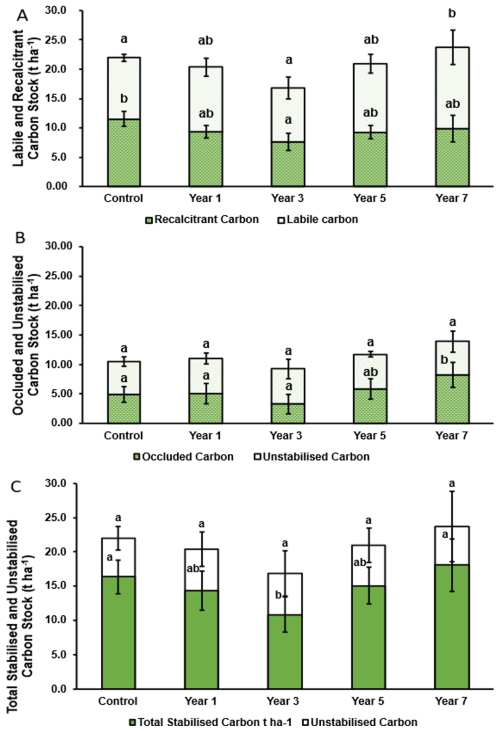
Figure 7Carbon stock (n= 5) split by thermally recalcitrant carbon (hashed) and thermally labile carbon (plain) (A) and occluded carbon (hashed) and unstabilised carbon (plain) (B); and total stabilised carbon (Green) and unstabilised carbon (plain). Total stabilised carbon considered both recalcitrant and occluded carbon stocks. Error bars represent ±1 SD. Dissimilar lower-case letters indicate significant (p≤ 0.05) differences across the timeseries.
Over the 7 year period recalcitrant carbon stock was observed to decrease (not significantly (p≥ 0.05) to 9.85 t C ha−1 (from 11.54 t C ha−1), while labile carbon stocks were observed to increase significantly (p≤ 0.05) to 13.87 t C ha−1 (from 10.44 t C ha−1). Highlighting that the overall net 1.75 t C ha−1 increase observed in soil carbon stock over the 7 year period was comprised entirely of labile carbon (Figs. 7A and S2).
While recalcitrant carbon stocks were observed to increase in later years, this rate of increase was less than that of the labile carbon pool (Fig. 7A). However, it is likely that recalcitrant carbon stocks would recover to the level of the control and possibly increase further with additional time under regenerative management. Furthermore, It is likely that the initial decreases observed in both labile and recalcitrant carbon pools related to soil disturbance and changing/reduction of organic input (crop residue) when initially transitioning from an arable to blackcurrant crop, alongside a soil priming effect from the increase in labile carbon content increasing the diversity and abundance of soil microbial communities that promote decomposition (De Graaff et al., 2010; Yazdanpanah et al., 2016; Lal et al., 2018; Amin et al., 2021). Indeed, it has been observed that significantly increasing labile carbon inputs to the soil can undermine the stability of recalcitrant carbon due to this enhanced priming effect (De Graaff et al., 2010), potentially causing the recalcitrant carbon loss initially observed.
Occluded carbon stocks were observed to increase marginally (not significant (p≥ 0.05)) between the control and year 1 soil (from 4.81 to 4.98 t C ha−1), before decreasing relative to both in the year 3 soil (not significantly (p≥ 0.05)) (to 3.23 t C ha−1) (Fig. 7B). Subsequently, occluded carbon stocks were observed to increase in the years 5 and 7 soils (to 5.82 t C ha−1 (not significantly (p≥ 0.05)), and 8.21 t C ha−1 (significantly (p≤ 0.05))). An overall significant (p≤ 0.05) increase in the occluded carbon pool between the control and year 7 soils, almost doubling from 4.81 to 8.21 t C ha−1 (Fig. 7B). While unstabilised carbon was observed to remain broadly consistent across all soils with no significant differences (p≥ 0.05) measured (Fig. 7B). Indeed, unstabilised carbon remained relatively unchanged between the control and year 7 soil (5.63 and 5.67 t C ha−1 respectively). However, a small increase was observed in the year 1 soil following cultivation, increasing to 6.02 t C ha−1, before converging (Fig. 7B). It is highlighted that the significant (p≤ 0.05) increase in occluded carbon corresponds to the almost identical increase in labile carbon measured in the same time period (3.40 and 3.42 t C ha−1 respectively) (Fig. 7A/B). As such, it can be concluded that virtually all the uplift in labile carbon measured over the 7 year period had been physically protected within the stable aggregate fraction as occluded carbon. This result is important as it confirms regenerative practices have been effective in cultivating aggregate stabilities capable of physically protecting what would otherwise be potentially degradable, labile, carbon. Thus, when viewed as total stabilised carbon (inclusive of recalcitrant carbon and occluded carbon) a total 1.7 t C ha−1 increase (not significant (p≥ 0.05) of potentially sequesterable carbon was observed after 7 years of regenerative management relative to the control (Fig. 7C).
3.7 Carbon sequestration
Efforts to increase soil carbon stocks, through methods such as regenerative agriculture, have become increasingly important strategies to support climate change mitigation (Lal, 1997, 2004; Lal et al., 2004; Smith, 2008; Smith et al., 2020; Soussana et al., 2019; Baveye et al., 2020; Keenor et al., 2021). However, it is important that we acknowledge not all carbon is equal in terms of its long-term sequestration potential: The results presented herein highlight the important nuances of both recalcitrant carbon pools and the physical protection of carbon (labile and/or recalcitrant) within soil aggregates. Given the physical protection conferred by stable soil aggregates even relatively labile carbon structures may be stabilised and physically protected in the long term as a result of their occlusion from degradative forces; with the aggregate stability governing the carbon residence time rather than its inherent stability (Schrumpf et al., 2013; Gärdenäs et al., 2011; Dungait et al., 2012; Six and Jastrow, 2002; Plante et al., 2011; McLauchlan and Hobbie, 2004) (Sect. 3.4, 3.5). While the average mean residence time of aggregate stabilised carbon can range from decades to centuries, similarly to that of recalcitrant carbon, the permanence of this carbon can vary greatly between different land use types (as a result of soil management practice) (Six and Jastrow, 2002; Rabbi et al., 2013). As such It is highlighted that carbon protection is only conferred for as long as the carbon is occluded – i.e. activities that damage and destroy soil aggregates (soil disturbance and ploughing) can reverse these physical protections and allow for the re-entry of this carbon to the degradative labile carbon pool from which it had previously been isolated (Pandey et al., 2014; Six et al., 1998; McLauchlan and Hobbie, 2004). Within a no till rotational system, carbon storage within stable aggregates has been observed to range between 27–137 years (Six and Jastrow, 2002). Thus providing significant means of stabilising and sequestering carbon in the medium- to long-term, within regeneratively managed systems (Lal, 1997; Abiven et al., 2009), and potentially on par with that of recalcitrant carbon stocks (Mao et al., 2022).
Additionally, for accurate carbon sequestration accounting to be realised, focus must be placed on the role soil bulk density plays in carbon sequestration calculations; as changes in soil carbon content often culminate in commensurate changes to the bulk density of a soil (Ruehlmann and Körschens, 2009; Smith et al., 2020; Rovira et al., 2022). Simply, as soil bulk density changes, the total volume that the soil occupies also changes (the total amount of soil remains the same, but its structure and arrangement in 3D space does not). Where soil bulk density decreases, the mass of soil per unit volume decreases. Consequently, to increase field-scale carbon stocks (assessed to a prescribed depth), SOC (g kg−1) must increase at a greater rate than bulk density decreases.
In this research, SBD (Sect. 3.1), was observed to decrease in the top 7.5 cm with increased time under regenerative agricultural management practices, meanwhile soil carbon content (Sect. 3.2) was observed to increase with time. However, when changes in carbon stocks were considered on a t C ha−1 basis (with the prescribed soil depth of 7.5 cm), carbon stocks did not increase incrementally with increasing time (Sect. 3.6; Fig. S2). In effect there was a trade-off, as the rate of SBD decrease outpaced that of SOC increase. Consequentially, where soil carbon stocks are considered, while carbon content of the soil increased by ∼ 65 % over the 7 year period (increasing from 16.6 g kg−1 in the control to 27.5 g kg−1 after 7 years (alley and bush soil collectively)), the total field scale increase in carbon stock was only ∼ 8 % (increasing from 21.98 to 23.72 t ha−1) over the 7.5 cm depth measured (Fig. S2).
Our results highlight the antagonism that exists between SBD and SOC where a prescribed soil depth is applied to soil carbon stock calculations. Thus, it is arguably more appropriate to acknowledge the depth of horizon transitions within a soil profile, and where SBD is increasing (e.g. with time under regenerative practices) to in effect increase the volume of the original soil, this new soil depth of the horizon should be used in carbon stock calculation.
Yet it is often the case that soil analysis reports do not acknowledge these changes in SBD; rather they present absolute soil carbon content (%). As a consequence, the credibility of both on-farm emissions reductions and creation of soil carbon credits is undermined, creating low integrity carbon sequestration and may lead to the abandonment of potentially significant transitional technologies due to a lack of trust. As such, the standardisation of soil carbon accountancy methods, (alongside robust validation and verification) is imperative to restoring confidence and boosting the integrity of soil based carbon sequestration (Keenor et al., 2021).
Thus, accounting for recalcitrant carbon and total stabilised carbon with respect to the SBD measured, potentially sequesterable soil carbon was measured to increase over the 7 year period by 1.7 t C ha−1 (Sect. 3.6; Fig. 7C); offering significant benefit and potential to long term carbon storage at the farm and landscape scale. When calculated against the scale of regenerative blackcurrant production at Gorgate Farm (50.3 ha) a total potential of 314 t CO2e could be sequestered in the top 7.5 cm of soil over a 7 year period, with carbon residence on a decadal timescale.
As perennial plants, soft fruit and orchard crops offer significant opportunities for investment, engagement, and adoption of regenerative agriculture principles for soil enhancement and climate change mitigation, due to their low maintenance – long-term growing cycle and minimised soil disturbance. Were the same regenerative methods as practiced at Gorgate Farm to be applied to all UK soft fruit production (total of 10 819 ha, DEFRA, 2023), this could provide a total UK wide sequestration potential of 67 500 t CO2e (after 7 years of continuous management).
The results of this research highlight the potential for regenerative agriculture practices to increase SOC, increase the proportions of WSA, and physically protect labile carbon within these aggregates – thus affording opportunities for long-term carbon sequestration as stabilised soil carbon stocks. However, our results also bring to the fore important factors relating to soil carbon stock assessment. In particular, the antagonism between SBD decreasing at a rate greater than SOC increases; this creating a trade-off where soil carbon stocks are calculated to a standard prescribed depth, not an equivalent mass.
Thus, we highlight further research and practical guidance is needed to enable more robust soil carbon stock assessment that acknowledges (i) a full pedogenic soil horizon, (ii) further delineation of soil carbon pools (POM vs. MOAM) (iii) the inherent recalcitrance of SOC, and (iv) the proportion of SOC physically protected by association with soil aggregates.
Data can be made available from the corresponding author upon request.
The supplement related to this article is available online at https://doi.org/10.5194/soil-11-957-2025-supplement.
BJR was the Principal Investigator and SGK the Senior Researcher for this research. Together SGK, BJR and RL undertook the investigation planning and fieldwork. Laboratory work was led by SGK with assistance in preliminary laboratory study and WSA method development from RL. SGK undertook the soil data and carbon stability analysis, statistical analysis, literature review, and the drafting of the manuscript. SGK and BJR undertook review and editing to deliver the final manuscript.
The contact author has declared that none of the authors has any competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors. Views expressed in the text are those of the authors and do not necessarily reflect the views of the publisher.
This research was supported by the Natural Environment Research Council and ARIES DTP (grant no. NE/S007334/1) with additional support provided by Greenworld Sales Ltd, Norfolk.
This paper was edited by Katerina Georgiou and reviewed by Guusje Koorneef and two anonymous referees.
Abiven, S., Menasseri, S., and Chenu, C.: The effects of organic inputs over time on soil aggregate stability – A literature analysis, Soil Biology and Biochemistry, 41, 1–12, https://doi.org/10.1016/j.soilbio.2008.09.015, 2009.
Al-Kaisi, M. M. and Lal, R.: Aligning science and policy of regenerative agriculture, Soil Science Society of America Journal, 84, 1808–1820, https://doi.org/10.1002/saj2.20162, 2020.
Al-Shammary, A. A. G., Kouzani, A. Z., Kaynak, A., Khoo, S. Y., Norton, M., and Gates, W.: Soil Bulk Density Estimation Methods: A Review, Pedosphere, 28, 581–596, https://doi.org/10.1016/S1002-0160(18)60034-7, 2018.
Allen, D. E., Singh, B. P., and Dalal, R. C.: Soil Health Indicators Under Climate Change: A Review of Current Knowledge, in: Soil Health and Climate Change, edited by: Singh, B. P., Cowie, A. L., and Chan, K. Y., Springer Berlin Heidelberg, Berlin, Heidelberg, 25–45, https://doi.org/10.1007/978-3-642-20256-8_2, 2011.
Amin, M., Salamba, H. N., and Juita, N.: Role of labile fraction of carbon for soil quality assessment (A Review), IOP Conference Series: Earth and Environmental Science, 807, 032095, https://doi.org/10.1088/1755-1315/807/3/032095, 2021.
Baveye, P. C., Baveye, J., and Gowdy, J.: Soil “Ecosystem” Services and Natural Capital: Critical Appraisal of Research on Uncertain Ground, Frontiers in Environmental Science, 4, https://doi.org/10.3389/fenvs.2016.00041, 2016.
Baveye, P. C., Schnee, L. S., Boivin, P., Laba, M., and Radulovich, R.: Soil Organic Matter Research and Climate Change: Merely Re-storing Carbon Versus Restoring Soil Functions, Frontiers in Environmental Science, 8, https://doi.org/10.3389/fenvs.2020.579904, 2020.
Berhe, A. A. and Kleber, M.: Erosion, deposition, and the persistence of soil organic matter: mechanistic considerations and problems with terminology, Earth Surface Processes and Landforms, 38, 908-912, https://doi.org/10.1002/esp.3408, 2013.
Bhogal, A., Nicholson, F. A., and Chambers, B. J.: Organic carbon additions: effects on soil bio-physical and physico-chemical properties, European Journal of Soil Science, 60, 276–286, https://doi.org/10.1111/j.1365-2389.2008.01105.x, 2009.
Brodowski, S., John, B., Flessa, H., and Amelung, W.: Aggregate-occluded black carbon in soil, European Journal of Soil Science, 57, 539–546, https://doi.org/10.1111/j.1365-2389.2006.00807.x, 2006.
Chaplot, V. and Cooper, M.: Soil aggregate stability to predict organic carbon outputs from soils, Geoderma, 243–244, 205–213, https://doi.org/10.1016/j.geoderma.2014.12.013, 2015.
Chaudhari, P. R., Ahire, D. V., Ahire, V. D., Chkravarty, M., and Maity, S.: Soil bulk density as related to soil texture, organic matter content and available total nutrients of Coimbatore soil, International Journal of Scientific and Research Publications, 3, 1–8, 2013.
De Graaff, M. A., Classen, A. T., Castro, H. F., and Schadt, C. W.: Labile soil carbon inputs mediate the soil microbial community composition and plant residue decomposition rates, New Phytologist, 188, 1055–1064, 2010.
de Groot, R. S., Wilson, M. A., and Boumans, R. M. J.: A typology for the classification, description and valuation of ecosystem functions, goods and services, Ecological Economics, 41, 393–408, https://doi.org/10.1016/S0921-8009(02)00089-7, 2002.
De Gryze, S., Six, J., and Merckx, R.: Quantifying water-stable soil aggregate turnover and its implication for soil organic matter dynamics in a model study, European Journal of Soil Science, 57, 693–707, https://doi.org/10.1111/j.1365-2389.2005.00760.x, 2006.
DEFRA: Horticulture Statistics 2023, https://www.gov.uk/government/statistics/latest-horticulture-statistics/horticulture-statistics-2024 (last access: 21 August 2024), 2023.
Dell'Abate, M. T., Benedetti, A., and Sequi, P.: Thermal Methods of Organic Matter Maturation Monitoring During a Composting Process, J. Therm. Anal. Calorim., 61, 389–396, https://doi.org/10.1023/A:1010157115211, 2000.
Dell'Abate, M. T., Benedetti, A., and Brookes, P. C.: Hyphenated techniques of thermal analysis for characterisation of soil humic substances, Journal of Separation Science, 26, 433–440, https://doi.org/10.1002/jssc.200390057, 2003.
Dominati, E., Patterson, M., and Mackay, A.: A framework for classifying and quantifying the natural capital and ecosystem services of soils, Ecological Economics, 69, 1858–1868, https://doi.org/10.1016/j.ecolecon.2010.05.002, 2010.
Dungait, J. A. J., Hopkins, D. W., Gregory, A. S., and Whitmore, A. P.: Soil organic matter turnover is governed by accessibility not recalcitrance, Global Change Biology, 18, 1781–1796, https://doi.org/10.1111/j.1365-2486.2012.02665.x, 2012.
Ferreira, C. d. R., Silva Neto, E. C. d., Pereira, M. G., Guedes, J. d. N., Rosset, J. S., and Anjos, L. H. C. d.: Dynamics of soil aggregation and organic carbon fractions over 23 years of no-till management, Soil and Tillage Research, 198, 104533, https://doi.org/10.1016/j.still.2019.104533, 2020.
Foley, J. A., DeFries, R., Asner, G. P., Barford, C., Bonan, G., Carpenter, S. R., Chapin, F. S., Coe, M. T., Daily, G. C., Gibbs, H. K., Helkowski, J. H., Holloway, T., Howard, E. A., Kucharik, C. J., Monfreda, C., Patz, J. A., Prentice, I. C., Ramankutty, N., and Snyder, P. K.: Global Consequences of Land Use, Science, 309, 570–574, https://doi.org/10.1126/science.1111772, 2005.
Foley, J. A., Ramankutty, N., Brauman, K. A., Cassidy, E. S., Gerber, J. S., Johnston, M., Mueller, N. D., O'Connell, C., Ray, D. K., West, P. C., Balzer, C., Bennett, E. M., Carpenter, S. R., Hill, J., Monfreda, C., Polasky, S., Rockström, J., Sheehan, J., Siebert, S., Tilman, D., and Zaks, D. P. M.: Solutions for a cultivated planet, Nature, 478, 337–342, https://doi.org/10.1038/nature10452, 2011.
Gál, A., Vyn, T. J., Michéli, E., Kladivko, E. J., and McFee, W. W.: Soil carbon and nitrogen accumulation with long-term no-till versus moldboard plowing overestimated with tilled-zone sampling depths, Soil and Tillage Research, 96, 42–51, https://doi.org/10.1016/j.still.2007.02.007, 2007.
Gärdenäs, A. I., Ågren, G. I., Bird, J. A., Clarholm, M., Hallin, S., Ineson, P., Kätterer, T., Knicker, H., Nilsson, S. I., Näsholm, T., Ogle, S., Paustian, K., Persson, T., and Stendahl, J.: Knowledge gaps in soil carbon and nitrogen interactions – From molecular to global scale, Soil Biology and Biochemistry, 43, 702–717, https://doi.org/10.1016/j.soilbio.2010.04.006, 2011.
Giller, K. E., Hijbeek, R., Andersson, J. A., and Sumberg, J.: Regenerative agriculture: an agronomic perspective, Outlook on Agriculture, 50, 13–25, 2021.
Gregorich, E. G., Gillespie, A. W., Beare, M. H., Curtin, D., Sanei, H., and Yanni, S. F.: Evaluating biodegradability of soil organic matter by its thermal stability and chemical composition, Soil Biology and Biochemistry, 91, 182–191, https://doi.org/10.1016/j.soilbio.2015.08.032, 2015.
Hemming, P. E.: C,H,N MICRO-ANALYSIS: A COMPARATIVE REVIEW OF THE EFFECTS OF INSTRUMENT DESIGN ON ANALYTICAL PERFORMANCE, https://exeteranalytical.com/paper.pdf (last access: 14 Novemeber 2025), 2024.
Huang, J., Zhang, G., Zhang, Y., Guan, X., Wei, Y., and Guo, R.: Global desertification vulnerability to climate change and human activities, Land Degradation & Development, 31, 1380–1391, https://doi.org/10.1002/ldr.3556, 2020.
IPBES: The IPBES assessment report on land degradation and restoration, Zenodo, https://doi.org/10.5281/zenodo.3237393, 2018.
Kan, Z.-R., Liu, Q.-Y., Virk, A. L., He, C., Qi, J.-Y., Dang, Y. P., Zhao, X., and Zhang, H.-L.: Effects of experiment duration on carbon mineralization and accumulation under no-till, Soil and Tillage Research, 209, 104939, https://doi.org/10.1016/j.still.2021.104939, 2021.
Kasper, M., Buchan, G. D., Mentler, A., and Blum, W. E. H.: Influence of soil tillage systems on aggregate stability and the distribution of C and N in different aggregate fractions, Soil and Tillage Research, 105, 192–199, https://doi.org/10.1016/j.still.2009.08.002, 2009.
Kaufmann, M., Tobias, S., and Schulin, R.: Comparison of critical limits for crop plant growth based on different indicators for the state of soil compaction, Journal of Plant Nutrition and Soil Science, 173, 573–583, https://doi.org/10.1002/jpln.200900129, 2010.
Keenor, S. G., Rodrigues, A. F., Mao, L., Latawiec, A. E., Harwood, A. R., and Reid, B. J.: Capturing a soil carbon economy, Royal Society Open Science, 8, 202305, https://doi.org/10.1098/rsos.202305, 2021.
Kleber, M.: What is recalcitrant soil organic matter?, Environmental Chemistry, 7, 320–332, https://doi.org/10.1071/EN10006, 2010.
Lal, R.: Residue management, conservation tillage and soil restoration for mitigating greenhouse effect by CO2-enrichment, Soil and Tillage Research, 43, 81–107, https://doi.org/10.1016/S0167-1987(97)00036-6, 1997.
Lal, R.: Soil degradation by erosion, Land Degradation & Development, 12, 519–539, https://doi.org/10.1002/ldr.472, 2001.
Lal, R.: Soil carbon sequestration to mitigate climate change, Geoderma, 123, 1–22, https://doi.org/10.1016/j.geoderma.2004.01.032, 2004.
Lal, R.: Restoring Soil Quality to Mitigate Soil Degradation, Sustainability, 7, 5875–5895, 2015.
Lal, R., Griffin, M., Apt, J., Lave, L., and Morgan, M. G.: Managing Soil Carbon, Science, 304, 393–393, https://doi.org/10.1126/science.1093079, 2004.
Lal, R., Smith, P., Jungkunst, H. F., Mitsch, W. J., Lehmann, J., Nair, P. K. R., McBratney, A. B., Sá, J. C. d. M., Schneider, J., Zinn, Y. L., Skorupa, A. L. A., Zhang, H.-L., Minasny, B., Srinivasrao, C., and Ravindranath, N. H.: The carbon sequestration potential of terrestrial ecosystems, Journal of Soil and Water Conservation, 73, 145A–152A, https://doi.org/10.2489/jswc.73.6.145A, 2018.
Lambin, E. F., Turner, B. L., Geist, H. J., Agbola, S. B., Angelsen, A., Bruce, J. W., Coomes, O. T., Dirzo, R., Fischer, G., Folke, C., George, P. S., Homewood, K., Imbernon, J., Leemans, R., Li, X., Moran, E. F., Mortimore, M., Ramakrishnan, P. S., Richards, J. F., Skånes, H., Steffen, W., Stone, G. D., Svedin, U., Veldkamp, T. A., Vogel, C., and Xu, J.: The causes of land-use and land-cover change: moving beyond the myths, Global Environmental Change, 11, 261–269, https://doi.org/10.1016/S0959-3780(01)00007-3, 2001.
Lavallee, J. M., Soong, J. L., and Cotrufo, M. F.: Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century, Global Change Biology, 26, 261–273, https://doi.org/10.1111/gcb.14859, 2020.
Ledo, A., Smith, P., Zerihun, A., Whitaker, J., Vicente-Vicente, J. L., Qin, Z., McNamara, N. P., Zinn, Y. L., Llorente, M., Liebig, M., Kuhnert, M., Dondini, M., Don, A., Diaz-Pines, E., Datta, A., Bakka, H., Aguilera, E., and Hillier, J.: Changes in soil organic carbon under perennial crops, Global Change Biology, 26, 4158–4168, https://doi.org/10.1111/gcb.15120, 2020.
Lehmann, J., Bossio, D. A., Kögel-Knabner, I., and Rillig, M. C.: The concept and future prospects of soil health, Nature Reviews Earth & Environment, 1, 544–553, https://doi.org/10.1038/s43017-020-0080-8, 2020.
Mao, L., Keenor, S. G., Cai, C., Kilham, S., Murfitt, J., and Reid, B. J.: Recycling paper to recarbonise soil, Science of The Total Environment, 847, 157473, https://doi.org/10.1016/j.scitotenv.2022.157473, 2022.
Martin, T. and Sprunger, C. D.: Sensitive Measures of Soil Health Reveal Carbon Stability Across a Management Intensity and Plant Biodiversity Gradient, Frontiers in Soil Science, 2, https://doi.org/10.3389/fsoil.2022.917885, 2022.
McLauchlan, K. K. and Hobbie, S. E.: Comparison of Labile Soil Organic Matter Fractionation Techniques, Soil Science Society of America Journal, 68, 1616–1625, https://doi.org/10.2136/sssaj2004.1616, 2004.
Mikha, M. M., Jin, V. L., Johnson, J. M. F., Lehman, R. M., Karlen, D. L., and Jabro, J. D.: Land management effects on wet aggregate stability and carbon content, Soil Science Society of America Journal, 85, 2149–2168, https://doi.org/10.1002/saj2.20333, 2021.
Montanarella, L., Pennock, D. J., McKenzie, N., Badraoui, M., Chude, V., Baptista, I., Mamo, T., Yemefack, M., Singh Aulakh, M., Yagi, K., Young Hong, S., Vijarnsorn, P., Zhang, G.-L., Arrouays, D., Black, H., Krasilnikov, P., Sobocká, J., Alegre, J., Henriquez, C. R., de Lourdes Mendonça-Santos, M., Taboada, M., Espinosa-Victoria, D., AlShankiti, A., AlaviPanah, S. K., Elsheikh, E. A. E. M., Hempel, J., Camps Arbestain, M., Nachtergaele, F., and Vargas, R.: World's soils are under threat, SOIL, 2, 79–82, https://doi.org/10.5194/soil-2-79-2016, 2016.
Newton, P., Civita, N., Frankel-Goldwater, L., Bartel, K., and Johns, C.: What Is Regenerative Agriculture? A Review of Scholar and Practitioner Definitions Based on Processes and Outcomes, Frontiers in Sustainable Food Systems, 4, https://doi.org/10.3389/fsufs.2020.577723, 2020.
Nie, X., Li, Z., Huang, J., Liu, L., Xiao, H., Liu, C., and Zeng, G.: Thermal stability of organic carbon in soil aggregates as affected by soil erosion and deposition, Soil and Tillage Research, 175, 82–90, https://doi.org/10.1016/j.still.2017.08.010, 2018.
Ogle, S. M., Swan, A., and Paustian, K.: No-till management impacts on crop productivity, carbon input and soil carbon sequestration, Agriculture, Ecosystems & Environment, 149, 37–49, https://doi.org/10.1016/j.agee.2011.12.010, 2012.
Orgiazzi, A. and Panagos, P.: Soil biodiversity and soil erosion: It is time to get married, Global Ecology and Biogeography, 27, 1155–1167, https://doi.org/10.1111/geb.12782, 2018.
Pandey, D., Agrawal, M., Singh Bohra, J., Adhya, T. K., and Bhattacharyya, P.: Recalcitrant and labile carbon pools in a sub-humid tropical soil under different tillage combinations: A case study of rice–wheat system, Soil and Tillage Research, 143, 116–122, https://doi.org/10.1016/j.still.2014.06.001, 2014.
Paustian, K., Chenu, C., Conant, R., Cotrufo, F., Lal, R., Smith, P., and Soussana, J.-F.: Climate mitigation potential of regenerative agriculture is significant!, Regenerative Agriculture Foundation June, CCSD, https://static1.squarespace.com/static/67d45c6c013eb5317044a558/t/6830e4a56bda5009073448ec/1748034725418/Climate<BMitigation>Potential<Bof>BRegenerative<Ag>is<Significant>-<Response>to<WRI.pdf (last access 14 March 2024), 2020.
Pearson, C. J.: Regenerative, Semiclosed Systems: A Priority for Twenty-First-Century Agriculture, BioScience, 57, 409–418, https://doi.org/10.1641/b570506, 2007.
Penman, J., Gytarsky, M., Hiraishi, T., Krug, T., Kruger, D., Pipatti, R., Buendia, L., Miwa, K., Ngara, T., and Tanabe, K.: Good practice guidance for land use, land-use change and forestry, Institute for Global Environmental Strategies, http://www.ipcc-nggip.iges.or.jp/public/gpglulucf.html, 2003.
Pittelkow, C. M., Linquist, B. A., Lundy, M. E., Liang, X., van Groenigen, K. J., Lee, J., van Gestel, N., Six, J., Venterea, R. T., and van Kessel, C.: When does no-till yield more? A global meta-analysis, Field Crop Res., 183, 156–168, https://doi.org/10.1016/j.fcr.2015.07.020, 2015.
Plante, A. F., Pernes, M., and Chenu, C.: Changes in clay-associated organic matter quality in a C depletion sequence as measured by differential thermal analyses, Geoderma, 129, 186–199, https://doi.org/10.1016/j.geoderma.2004.12.043, 2005.
Plante, A. F., Fernández, J. M., Haddix, M. L., Steinweg, J. M., and Conant, R. T.: Biological, chemical and thermal indices of soil organic matter stability in four grassland soils, Soil Biology and Biochemistry, 43, 1051–1058, https://doi.org/10.1016/j.soilbio.2011.01.024, 2011.
Power, A. G.: Ecosystem services and agriculture: tradeoffs and synergies, Philosophical Transactions of the Royal Society B: Biological Sciences, 365, 2959–2971, https://doi.org/10.1098/rstb.2010.0143, 2010.
Rabbi, S. M. F., Hua, Q., Daniel, H., Lockwood, P. V., Wilson, B. R., and Young, I. M.: Mean Residence Time of Soil Organic Carbon in Aggregates Under Contrasting Land Uses Based on Radiocarbon Measurements, Radiocarbon, 55, 127–139, https://doi.org/10.2458/azu_js_rc.v55i1.16179, 2013.
Rieke, E. L., Bagnall, D. K., Morgan, C. L. S., Flynn, K. D., Howe, J. A., Greub, K. L. H., Mac Bean, G., Cappellazzi, S. B., Cope, M., Liptzin, D., Norris, C. E., Tracy, P. W., Aberle, E., Ashworth, A., Bañuelos Tavarez, O., Bary, A. I., Baumhardt, R. L., Borbón Gracia, A., Brainard, D. C., Brennan, J. R., Briones Reyes, D., Bruhjell, D., Carlyle, C. N., Crawford, J. J. W., Creech, C. F., Culman, S. W., Deen, B., Dell, C. J., Derner, J. D., Ducey, T. F., Duiker, S. W., Dyck, M. F., Ellert, B. H., Entz, M. H., Espinosa Solorio, A., Fonte, S. J., Fonteyne, S., Fortuna, A.-M., Foster, J. L., Fultz, L. M., Gamble, A. V., Geddes, C. M., Griffin-LaHue, D., Grove, J. H., Hamilton, S. K., Hao, X., Hayden, Z. D., Honsdorf, N., Ippolito, J. A., Johnson, G. A., Kautz, M. A., Kitchen, N. R., Kumar, S., Kurtz, K. S. M., Larney, F. J., Lewis, K. L., Liebman, M., Lopez Ramirez, A., Machado, S., Maharjan, B., Martinez Gamiño, M. A., May, W. E., McClaran, M. P., McDaniel, M. D., Millar, N., Mitchell, J. P., Moore, A. D., Moore, P. A., Mora Gutiérrez, M., Nelson, K. A., Omondi, E. C., Osborne, S. L., Osorio Alcalá, L., Owens, P., Pena-Yewtukhiw, E. M., Poffenbarger, H. J., Ponce Lira, B., Reeve, J. R., Reinbott, T. M., Reiter, M. S., Ritchey, E. L., Roozeboom, K. L., Rui, Y., Sadeghpour, A., Sainju, U. M., Sanford, G. R., Schillinger, W. F., Schindelbeck, R. R., Schipanski, M. E., Schlegel, A. J., Scow, K. M., Sherrod, L. A., Shober, A. L., Sidhu, S. S., Solís Moya, E., St. Luce, M., Strock, J. S., Suyker, A. E., Sykes, V. R., Tao, H., Trujillo Campos, A., Van Eerd, L. L., van Es, H. M., Verhulst, N., Vyn, T. J., Wang, Y., Watts, D. B., Wright, D. L., Zhang, T., and Honeycutt, C. W.: Evaluation of aggregate stability methods for soil health, Geoderma, 428, 116156, https://doi.org/10.1016/j.geoderma.2022.116156, 2022.
Rovira, P., Sauras-Yera, T., and Romanyà, J.: Equivalent-mass versus fixed-depth as criteria for quantifying soil carbon sequestration: How relevant is the difference?, CATENA, 214, 106283, https://doi.org/10.1016/j.catena.2022.106283, 2022.
Ruehlmann, J. and Körschens, M.: Calculating the Effect of Soil Organic Matter Concentration on Soil Bulk Density, Soil Science Society of America Journal, 73, 876–885, https://doi.org/10.2136/sssaj2007.0149, 2009.
Sanderman, J., Hengl, T., and Fiske, G. J.: Soil carbon debt of 12,000 years of human land use, Proceedings of the National Academy of Sciences, 114, 9575–9580, https://doi.org/10.1073/pnas.1706103114, 2017.
Schmidt, M. W. I., Torn, M. S., Abiven, S., Dittmar, T., Guggenberger, G., Janssens, I. A., Kleber, M., Kögel-Knabner, I., Lehmann, J., Manning, D. A. C., Nannipieri, P., Rasse, D. P., Weiner, S., and Trumbore, S. E.: Persistence of soil organic matter as an ecosystem property, Nature, 478, 49–56, https://doi.org/10.1038/nature10386, 2011.
Schrumpf, M., Kaiser, K., Guggenberger, G., Persson, T., Kögel-Knabner, I., and Schulze, E.-D.: Storage and stability of organic carbon in soils as related to depth, occlusion within aggregates, and attachment to minerals, Biogeosciences, 10, 1675–1691, https://doi.org/10.5194/bg-10-1675-2013, 2013.
Seybold, C. A. and Herrick, J. E.: Aggregate stability kit for soil quality assessments, CATENA, 44, 37–45, https://doi.org/10.1016/S0341-8162(00)00175-2, 2001.
Shaheb, M. R., Venkatesh, R., and Shearer, S. A.: A Review on the Effect of Soil Compaction and its Management for Sustainable Crop Production, Journal of Biosystems Engineering, 46, 417–439, 10.1007/s42853-021-00117-7, 2021.
Sisti, C. P. J., dos Santos, H. P., Kohhann, R., Alves, B. J. R., Urquiaga, S., and Boddey, R. M.: Change in carbon and nitrogen stocks in soil under 13 years of conventional or zero tillage in southern Brazil, Soil and Tillage Research, 76, 39–58, https://doi.org/10.1016/j.still.2003.08.007, 2004.
Six, J. and Jastrow, J. D.: Organic matter turnover, Encyclopedia of soil Science, 10, 936–942, 2002.
Six, J., Elliott, E. T., Paustian, K., and Doran, J. W.: Aggregation and Soil Organic Matter Accumulation in Cultivated and Native Grassland Soils, Soil Science Society of America Journal, 62, 1367–1377, https://doi.org/10.2136/sssaj1998.03615995006200050032x, 1998.
Six, J., Bossuyt, H., Degryze, S., and Denef, K.: A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics, Soil and Tillage Research, 79, 7–31, https://doi.org/10.1016/j.still.2004.03.008, 2004.
Smith, P.: Land use change and soil organic carbon dynamics, Nutrient Cycling in Agroecosystems, 81, 169–178, https://doi-org.uea.idm.oclc.org/10.1007/s10705-007-9138-y, 2008.
Smith, P., Bustamante, M., Ahammad, H., Clark, H., Dong, H., Elsiddig, E. A., Haberl, H., Harper, R., House, J., and Jafari, M.: Agriculture, forestry and other land use (AFOLU), in: Climate change 2014: mitigation of climate change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, 811–922,https://www.ipcc.ch/site/assets/uploads/2018/02/ipcc_wg3_ar5_chapter11.pdf (last access: 16 June 2024), 2014.
Smith, P., Soussana, J.-F., Angers, D., Schipper, L., Chenu, C., Rasse, D. P., Batjes, N. H., van Egmond, F., McNeill, S., Kuhnert, M., Arias-Navarro, C., Olesen, J. E., Chirinda, N., Fornara, D., Wollenberg, E., Álvaro-Fuentes, J., Sanz-Cobena, A., and Klumpp, K.: How to measure, report and verify soil carbon change to realize the potential of soil carbon sequestration for atmospheric greenhouse gas removal, Global Change Biology, 26, 219–241, https://doi.org/10.1111/gcb.14815, 2020.
Soussana, J.-F., Lutfalla, S., Ehrhardt, F., Rosenstock, T., Lamanna, C., Havlík, P., Richards, M., Wollenberg, E., Chotte, J.-L., Torquebiau, E., Ciais, P., Smith, P., and Lal, R.: Matching policy and science: Rationale for the “4 per 1000 – soils for food security and climate” initiative, Soil and Tillage Research, 188, 3–15, https://doi.org/10.1016/j.still.2017.12.002, 2019.
Sundström, J. F., Albihn, A., Boqvist, S., Ljungvall, K., Marstorp, H., Martiin, C., Nyberg, K., Vågsholm, I., Yuen, J., and Magnusson, U.: Future threats to agricultural food production posed by environmental degradation, climate change, and animal and plant diseases – a risk analysis in three economic and climate settings, Food Security, 6, 201–215, https://doi.org/10.1007/s12571-014-0331-y, 2014.
Tilman, D., Balzer, C., Hill, J., and Befort, B. L.: Global food demand and the sustainable intensification of agriculture, Proceedings of the National Academy of Sciences, 108, 20260–20264, 2011.
Topa, D., Cara, I. G., and Jităreanu, G.: Long term impact of different tillage systems on carbon pools and stocks, soil bulk density, aggregation and nutrients: A field meta-analysis, CATENA, 199, 105102, https://doi.org/10.1016/j.catena.2020.105102, 2021.
Veenstra, J. L., Cloy, J. M., and Menon, M.: Physical and Hydrological Processes in Soils Under Conservation Tillage in Europe, in: Conservation Agriculture: A Sustainable Approach for Soil Health and Food Security: Conservation Agriculture for Sustainable Agriculture, edited by: Jayaraman, S., Dalal, R. C., Patra, A. K., and Chaudhari, S. K., Springer Singapore, Singapore, 391–406, https://doi.org/10.1007/978-981-16-0827-8_19, 2021.
Whalen, J. K., Hu, Q., and Liu, A.: Compost Applications Increase Water-Stable Aggregates in Conventional and No-Tillage Systems, Soil Science Society of America Journal, 67, 1842–1847, https://doi.org/10.2136/sssaj2003.1842, 2003.
Yazdanpanah, N., Mahmoodabadi, M., and Cerdà, A.: The impact of organic amendments on soil hydrology, structure and microbial respiration in semiarid lands, Geoderma, 266, 58–65, https://doi.org/10.1016/j.geoderma.2015.11.032, 2016.
Zika, M. and Erb, K.-H.: The global loss of net primary production resulting from human-induced soil degradation in drylands, Ecological Economics, 69, 310–318, https://doi.org/10.1016/j.ecolecon.2009.06.014, 2009.




