the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Benchmarking soil multifunctionality
Healthy soils provide multiple functions that contribute importantly to human wellbeing, including in primary production, climate and water regulation, and supporting biodiversity. These functions can partially be combined, and some functions also clearly trade off: this motivates soil multifunctionality research. Society needs scientists to help assess which soils are best for which soil functions and to determine appropriate long-term management of any given soil for optimal function delivery. However, for both tasks science lacks coherent tools, and, in this paper, I propose a way forward.
Critically, we lack a common measurement framework that pins soil functioning measurements on a common scale. Currently the field is divided with respect to the methods we use to measure and assess soil functioning and indicators thereof. Only three indicator variables (soil organic matter (SOM), acidity, and available P) were commonly measured (> 70 % of schemes) across 65 schemes that aim to measure soil health or quality, and no biological measure is implemented in more than 30 % of the 65 schemes. This status quo prevents us from systematically comparing across and within soils; we lack a soil multifunctionality benchmark.
We can address these limitations systematically by setting a common measurement system. To do this, I propose to use latent-variable modelling, based on a common set of functional measurements, to develop a common “IQ test for soils”. I treat soil functions as latent variables; because they are complex processes that cannot be measured directly, we can only detect drivers and consequences of these complex processes. Latent-variable modelling has a long history in social, economic, and psychometric fields, where it is known as factor analysis. Factor analysis aims to derive common descriptors – the factors – of hypothesized constructs by linking measurable response variables together on a common scale.
Here, I explain why such a new approach to soil multifunctionality and soil health is needed and how it can be operationalized. The framework developed here is an initial proposal; the issue of soil multifunctionality is too complex and too important to be addressed in one go. It needs to be resolved iteratively by groups of scientist working intensively together. We need to bring our best scientists together, in a collaborative effort, to develop progressively more refined ways of sustainably managing one of humanity's most precious resources: our soils.
- Article
(2965 KB) - Full-text XML
-
Supplement
(648 KB) - BibTeX
- EndNote
Human actions are perturbing the Earth system beyond its planetary boundaries, particularly in terms of biodiversity, climate, and flows of phosphorus and nitrogen, while we also need to provide sustainable social livelihoods across the globe (Fanning et al., 2022; Lade et al., 2020; Steffen et al., 2015). Agricultural production is a main driver of environmental problems due to land use change, depletion of freshwater resources, and pollution of aquatic and terrestrial ecosystems (Springmann et al., 2018). In addition, modern agriculture will have to adapt to global limits on mineral phosphorus supply (Blackwell et al., 2019) and increasing regulation of pesticide use (Tang and Maggi, 2021). This means land-bound agriculture will have to increasingly rely on the internal functional capacity of soils – e.g. to recycle nutrients and suppress diseases – and, thus, on soil health. Likewise, regulation of the climate through carbon sequestration and reducing greenhouse gas emissions (Lehmann et al., 2020) and the provision of habitats for aboveground biodiversity to bend the curve of biodiversity loss (Leclère et al., 2020) are directly and indirectly linked to soil health. Furthermore, soil biodiversity contributes importantly to climate change adaptation by facilitating water storage in soils through modifying soil organic matter (Lal, 2020) and achieving ONE Health through removal of contaminants and by preventing disease spread (Wall et al., 2015). Indeed, soil and soil health are at the heart of achieving many of the UN Sustainable Development Goals for 2030 (Keesstra et al., 2016; Lal et al., 2021) and the European Green Deal (Montanarella and Panagos, 2021).
Soil health, defined here as the continuing capacity of soils to deliver the multiple soil functions on which society depends, takes centre stage in policy and practice with respect to soils worldwide (Van der Putten et al., 2023; Veerman et al., 2020), and I use the term interchangeably with soil multifunctionality. However, currently the field is divided with respect to the methods we use to measure and assess soil functioning and indicators thereof. Only three indicator variables (soil organic matter (SOM), acidity, and available P) were commonly measured (> 70 % of schemes) across 65 schemes that aim to measure soil health or quality, and no biological measure is implemented in more than 30 % of the 65 schemes (Bünemann et al., 2018). Indeed, until very recently, there was no national- or European-level monitoring system that could address the key functions of soils comprehensively (Creamer et al., 2022; Van Leeuwen et al., 2017), although steps in this direction are now being taken (Norris et al., 2020; Orgiazzi et al., 2022; Zwetsloot et al., 2021), for instance in the EU's Soil Health Benchmarks project (https://soilhealthbenchmarks.eu, last access: 20 May 2025). It is clear that further harmonization in methods and quantification is urgently needed.
Partly, I think this plethora of methods and approaches stems from an oversimplified, often correlational understanding of the causal linkages driving soil multifunctionality, equipment availability in the laboratories involved, and a decades-old policy pressure to deliver easy-to-implement indicators fast (Creamer et al., 2022), which prevented the zooming-out needed to better understand the soil systematically (Harris et al., 2022). Indeed, what we need are “new analytical and conceptual approaches … that capture systems characteristics of soil health, in order to operationalize … monitoring soil health” (Lehmann et al., 2020). However, systemic perspectives that integrate soil functions and responses are in their infancy (Vogel et al., 2018). It is unclear how to manage the soil functions (Baveye et al., 2016) and how to link functions to soil processes (Vogel et al., 2018, but see Creamer et al., 2022). Integrating all soil processes is highly complex because soil properties are spatially heterogenous, and the interactions in soil are typically non-linear (Vogel et al., 2018). Soil biology is a key missing ingredient, and its complexity is paralysing the soil health literature (Creamer et al., 2022; Lehmann et al., 2020; Van Leeuwen et al., 2017). We know that soil biodiversity drives soil multifunctionality (Delgado-Baquerizo et al., 2016; Wagg et al., 2014), but the causal relation to soil functioning for many organisms is not clear (Creamer et al., 2022). Many soil microbial variables measured are hard to interpret and are insufficiently benchmarked to allow inferences about soil health (Fierer et al., 2021). Furthermore, most research focusses on soil health in an agricultural context (Debeljak et al., 2019; Fierer et al., 2021), but we also need to understand and quantify it in forestry, nature management, drinking-water production areas, and industrial and urban areas, which are strongly underrepresented (Norris et al., 2020; Orgiazzi et al., 2022).
To move forward, we first need to know what kind of information society needs from soil science. In this context, I think the main research tasks are as follows:
-
Determine which soils are best for which function (FAO and ITPS, 2015) and which functions can be combined (synergies) and which cannot (trade-offs).
-
Determine the functional shape of the interrelations among soil functions.
-
Determine the mechanistic drivers of the multiple functions of soils over a long-term timescale.
-
Determine how multifunctionality of individual soils can be optimized.
-
Develop a simple and effective indicator set to monitor the status and trends of soil functions and multifunctionality.
When we know these, we can start the spatial optimization of multifunctional soil use (van Wijnen et al., 2012), and if we understand the long-term impacts and dynamics with respect to the functions and their drivers, we can do so for long-term sustainable use.
To do these tasks well, we need to get organized as a scientific community. First and foremost, we need to set a common measurement system for the multiple functions of soil. We need a balanced set of indicators that reflect soil biology, chemistry, and physics but that are geared towards soil functioning (Lehmann et al., 2020). So far, selection of soil biological indicators was driven by well-known methods, feasibility in general laboratories, and costs, but this should be based on a sound understanding of how the indicators link to soil functioning mechanistically (Creamer et al., 2022; Lehmann et al., 2020; Vogel et al., 2018). New proposals typically try to go from soil processes to functions in one go, but soil is complex (Young and Crawford, 2004), and, so far, this approach has been defeated by this complexity. In many cases, the drivers of soil functions, either direct or indirect, are used implicitly or explicitly as proxies for the functions themselves. For example, soil nutrient content is used as a proxy for soil fertility (Daou and Shipley, 2019), and microbial biomass is used as a proxy for carbon storage (Wiesmeier et al., 2019); in both cases, these do contribute to the function but are not nearly a complete description of it. We can take steps forward by formally separating the causes and consequences and/or the predictors and the indicators of soil functioning and by linking them to the underlying processes and environmental and management context. I propose that we can do so by applying latent-variable models and structural causal modelling to soil multifunctionality research.
My aim with this paper is to propose a new methodology for measuring soil functioning and soil multifunctionality. It is based on the well-established technique of latent-variable modelling commonly used in psychometry, economics, and the social sciences at large. In parallel to my work presented here, Maaz et al. (2023) have also used latent-variable models to represent soil health; however, our approaches are quite distinct. They rely on a mixture of stocks, environmental conditions, and properties as indicators for soil health, while my aim is to link to the soil functions themselves. The next step after setting a valid measurement framework will be to develop a causal model of how trade-offs and synergies among soil functions are mechanistically regulated. If we define soil health as the continuing capacity of soils to deliver the multiple soil functions on which society depends then what are soil functions? Here, I define soil functions as soil processes, physical, chemical, or biological in nature, acting singly or in combination. These functions can be beneficial for human society but can also be involved in the internal functioning of ecosystems without direct human benefits, i.e. soil functioning for the sake of the ecosystem itself. For consistency, perhaps “soil functions on which society depends” should be called “soil services” as a specific form of ecosystem services.
Great mathematical frameworks now exist to combine multiple functions into one aggregate measure of multifunctionality (Byrnes et al., 2014, 2023), and they could be used to signal that “something is wrong” with soil functioning. However, understanding which soils perform all functions best in aggregate, e.g. the highest average soil function, is not informative enough to guide sustainable use of soils (Bradford et al., 2014; Lehmann et al., 2020). We need to know which soils perform which functions well and to what extend the functions can be combined or not in a single soil. So instead of focussing on univariate summary statistics of multifunctionality, we need to come up with a multivariate, but still simple and communicable, representation for soil multifunctionality (Lehmann et al., 2020; Zwetsloot et al., 2021). Multivariate models of multifunctionality have been developed, including network approaches that can be valuable in exploratory investigations (Siwicka et al., 2021). Others developed elegant multivariate models to estimate the influence of different drivers on functions and interrelations among functions (Dooley et al., 2015). However, all these approaches are correlational in nature, leaving the causal relationships that induced these correlations potentially unexamined (Shipley, 2016). I think this is problematic, because of (1) potential paradoxes in the data that no amount of big data can resolve (e.g. Simpson's paradox) and (2) difficulties in generalizing the results of analyses to other contexts. Posing a mechanistic model that links soil functions a priori, which is iteratively improved in the face of new data, can resolve both of these issues. In addition, hypothesizing about such mechanistic models will help in stabilizing the set of measured “functions” now rampant in the literature by excluding those indicators that are actually stocks or ecosystem properties and not processes (Garland et al., 2021; Lehmann et al., 2020). Confronting the hypothesized models with data and proposing improvements can be done with structural equation modelling (Box 1). However, how should the complexity of soils and soil functioning be organized into one model?
“Correlation is not causation” is a central piece of endemic wisdom we scientists throw at one another on a regular basis. However, its complement, “causation implies correlation”, is much less known due to Karl Pearson's (Pearson, 1911) crusade on causality. Nevertheless, it is the central concept in modern causal analysis (Pearl, 2009; Shipley, 2016). The modern causal revolution arose from the pioneering work of population geneticist Sewall Wright, who developed path analysis (Wright, 1921, 1934), a method to estimate causal effects from observational data. His method was ignored by statisticians and biologists for decades because it did not fit with the views of the dominant schools of statistics headed by Karl Pearson and Ronald A. Fisher (Shipley, 2016). Instead, the method was refined within economics, sociology, political science, and psychology (e.g. Jöreskog, 1967).
Path analysis was transformed into structural equation modelling (SEM), which uses maximum likelihood (ML) estimation to test causal multivariate hypotheses. The multivariate hypotheses are specified as a graph, specifically a directed acyclical graph, which captures the hypothesized causal relationships among the variables involved. The central idea is beautifully simple: if the specified causal hypothesis is true then we can predict which variables should be correlated and which should not be; the latter are considered to be conditionally independent. In fact, the method depends on predicting the covariance matrix of the variables, comparing it to the observed covariance matrix and testing the model fit (using an ML χ2 test). If the model does not fit the data (e.g. χ2p<0.05) then the hypothesized causal graph is rejected. If there is no lack of fit then one concludes that the data are consistent with the causal processes hypothesized (until, in the next paper, someone else proves you wrong, of course). For SEM to work, it needs to assume linear relationships and multivariate normal distributions of the variables involved, but it comes with the major advantage that it can estimate latent variables. Latent variables (LVs) are variables that were not measured or even cannot be measured. LVs are a way to measure the unmeasurable!
LVs are extremely important concepts as many things cannot be measured (Shipley, 2016). For instance, we cannot measure air temperature, which is the average kinetic energy of the molecules in the air; we can only measure its effects on, for example, the expansion of mercury in a capillary column (a mercury thermometer) or the change in electrical voltage in a thermocouple. These observed variables are of course causally linked to the latent quantity temperature, but they are observed with measurement error. Misspecifying this dependence relation in a causal model, thus conflating air temperature (“heat”) with the readings of your thermometer (translated to °C), can lead to an erroneous test of the causal model because it leads to a different expected covariance structure and, thus, different conditional independence claims. Latent-variable models (LVMs) are a way to get around this problem by specifying that the observed variable (thermocouple voltage) is caused by the latent quantity of interest (air temperature), but it is observed with error and therefore correlated but not identical. This situation is treated by “measurement models” (Fig. 1), a subsection of LVMs developed in the social sciences. To parameterize and test a single LV, four indicator variables need to be measured to have sufficient degrees of freedom, although this can be relaxed if the model entails multiple causally related LVs. LVs are also used to represent more hypothetical variables; e.g. concepts such as genes, atoms, and intelligence are examples of latent variables. These examples are successful latent concepts; there are also problematic ones, such as “ether”. Choosing, developing, and justifying latent variables are, perhaps, the most difficult aspects of structural equation modelling.
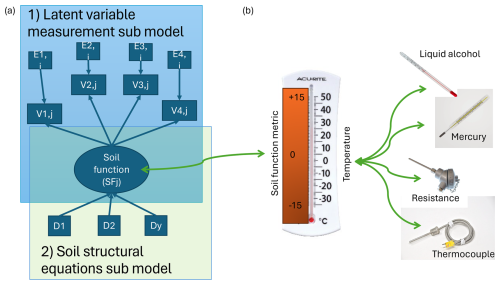
Figure 1The two parts of the full soil functioning model. (a) The two parts of the full soil functioning model including drivers (D1–Dy) and response variables (V1,j–V4,j), their error variances (Ei,j), and the latent variable representing a single soil function (SFj). See Box 1 for an introduction to structural equation modelling and latent-variable modelling. Part 1 concerns the latent-variable measurement submodel involving i indicators measured on each of the j soils for each soil function (SF). For example, in the case of primary production, the indicators are the growth responses (RGRij) of four different species used to estimate values for the latent variable of generalized soil fertility (FGj). The E's represent mutually independent measurement errors. See the Supplement for an implementation of the model on Dutch soil samples. Part 2 concerns the structural equation submodel. It consists of specifying the causal structure linking the y soil and non-soil variables and drivers (D1 to Dy) that cause SF. For soil fertility, for example, this could be NO3 concentration, water-holding capacity, and compaction. (b) Analogy of the soil function metrics to quantifying temperature of a waterbody as a latent variable using four differently operating thermometers. The latent “temperature” is estimated using a measurement model based on readings from a liquid-alcohol and a mercury thermometer based on column height measurements; a resistance thermometer, which responds to temperature by means of a change in electrical resistance; and a thermocouple, which responds to temperature by means of a change in electrical voltage. By combining these different measurements, a more accurate picture for temperature can be generated, given they are all adequate measures of temperature. Note, combining a good indicator with a poor indicator does not lead to improved accuracy; this is why indicators in LVMs need to be correlated to a good extent. This figure and the example are adapted from Daou and Shipley (2019).
Recently, the SEM toolbox was expanded with a new estimation and testing method based on d-separation. D-separation is a criterion used to derive conditional independence claims, specifying which variables should not be correlated given the a priori specified causal model (Shipley, 2000, 2016). The d-separation-based approach is flexible and can fully accommodate non-normal data, non-linear functional relationships, and nested sampling structures as it works not with the whole covariance matrix but instead looks at each d-separation independence claim separately (using partial correlations in its most simple form) and combines this to test the whole causal model using a Fisher's exact C test (C for combined, the d-sep test; Shipley, 2000). The logic is the same as for ML-based SEM. Given an a priori causal model, one tests for the conditional independence of variables predicted by the model.
Note, the methods of SEM and LVMs are implemented mathematically as regression models, but it is important to realize that the interpretation of SEM is much stronger than for ordinary regression models. Ordinary regression models are simple tools aiming only to predict the effect of X on Y. The goal is prediction, not primarily understanding, although the latter is often attempted. Causal interpretation of regression models is problematic because parameter estimates and significance depend strongly on the included variables and even their order. In fact, misrepresenting the underlying causal structure can easily lead to entirely the wrong qualitative conclusions, e.g. in the situation called Simpson's paradox (see the supplementary code at https://github.com/JasperWubs/SoilMFv0.1, last access: 20 May 2025), which no amount of data will resolve. SEM, however, is different. It is different not because of its mathematics; rather, it is different because it relies on an a priori causal hypothesis to be tested with data. The a priori is crucial: when SEM software is used to find the “best”-fitting model by means of model selection tools (e.g. the Akaike information criterion (AIC)) then Wright's philosophy falls apart, and SEM becomes just another regression tool only to be used for explorative data analysis and hypothesis generation. So, as an analyst using SEM, you get one, and only one, epistemologically sound shot at testing your causal hypothesis. Of course, upon arduously collecting data and then rejecting your model, there is immense temptation to update the model by including new, not a priori specified, causal relationships and to present the updated model in the resultant paper as if it were the original a priori model. This is a posteriori discovery and, again, is only suitable for exploration and hypothesis generation and not for direct causal interpretation. Therefore, I am strongly in favour of implementing a strict requirement that SEM used for causal hypothesis testing is preceded by the publication of the a priori model in a curated, time-stamped repository. Any updates to the model should be fully reported in the paper because newly discovered links require further testing. In this way, our causal models can be transparently developed and updated. For both SEM and LVMs, excellent textbooks, reviews, and manuals exist (see Grace, 2006; Grace et al., 2010, 2012; Shipley, 2016); this is also the case for other tools in the causal analysis toolkit (Pearl, 2009). This summary is a condensed version of the key points in Shipley (2016).
Before we can model interacting soil functions mechanistically, we need a common framework to measure them. For this we have to move beyond using simple indicators since the processes driving the different functions of soils are complex. Soil fertility, for instance, is a complex soil function that drives the process of primary production. It is complex because many factors contribute to it (Daou and Shipley, 2019), and it changes through time. Higher nutrient availability but also water content and soil texture and structure interact to shape how well plants grow in a soil. Furthermore, plant species and cultivars respond differently to the different drivers of soil fertility; for example, some prefer nitrate over ammonium, others are salt- or drought-tolerant, some can puncture compacted soils, and other species cannot (Grime, 2001). So, while it is possible to build a soil fertility model for individual crops by accounting for their limiting factors for growth and estimating the functional relationships to these factors, this is much more difficult to quantify in general with predictive values for all plant and crop species in a community simultaneously (Daou and Shipley, 2019).
Nevertheless, we can borrow the data analytic machinery used in the social sciences to estimate these complex soil traits. In psychology, economy, and other social disciplines, complex properties are measured using latent-variable models – specifically a subsection called “measurement models” – that allow an analyst to infer the status of the complex property by modelling the responses that the property induces (Fig. 1). A well-known example is the IQ test that aims to quantify the complex and hard-to-measure trait intelligence (Spearman, 1904). It does this by fitting a measurement model to the measurable outcomes of intelligence, namely a person's ability to solve particular puzzles in a limited time. Daou and Shipley have successfully adapted this methodology for quantifying generalized soil fertility (Daou et al., 2021; Daou and Shipley, 2019, 2020), and I propose that we expand their framework to include all major functions of soil so we can study soil multifunctionality more systematically; I propose an IQ test for soils.
Following the functional land management (FLM) framework (Debeljak et al., 2019; Schulte et al., 2014; Zwetsloot et al., 2021), I focus on four main soil functions of direct importance to society (Fig. 2). The IQ test for soils will focus on the following soil functions: (1) primary production, driven by soil fertility; (2) climate regulation, consisting of carbon storage and reducing greenhouse gas (GHG) emissions (or net GHG consumption by soil); (3) water regulation, composed of water storage and purification of contaminants; and, finally, (4) provision of habitat for biodiversity, focussing initially on plant diversity. See proposals for expansion to other species groups in the Discussion section. I exclude nutrient cycling, which is included in the FLM, because I think it is not a soil function beneficial to society in and of itself. Instead, I see it as a structuring principle; nutrient cycling determines where nutrients are “invested” and thus which functions “thrive” (see also Schröder et al., 2016). Additionally, direct issues with nutrients for society, e.g. low soil fertility and nitrate leaching, are captured under the other soil functions – respectively, primary production and water purification in these examples.
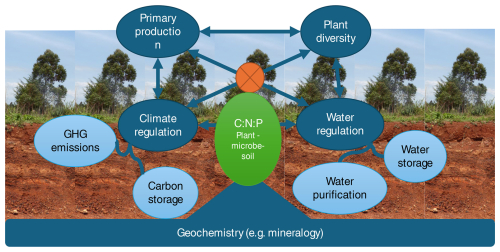
Figure 2Soils support human wellbeing in four main areas (blue circles), excluding direct and indirect contributions to human health.
Climate and water regulation are, respectively, further divided into the carbon storage and reducing greenhouse gas (GHG) emission subfunctions and water storage and purification subfunctions (light-blue circles) because of the very different causal mechanisms in play. The four soil functions are all interrelated – some trading off and others acting in synergy – because they all depend on the same basic resources (nutrients, energy, water). I hypothesize that the soil's plant–microbe–soil stoichiometry (green oval with orange operator sign) determines which functions are preferentially expressed by any given soil. How this regulation plays out is conditional on the geochemistry of the soil, mainly its mineralogy. Measuring the functions on a common scale and studying their interrelations using a common causal framework will help us determine how to manage soils for optimal multifunctionality.
The FLM framework was originally designed to integrate over relatively large spatial scales (Schreefel et al., 2022; Schulte et al., 2014) and uses decision trees, partly based on expert judgement, to generate assessments of the different soil functions on a semi-quantitative scale (low–medium–high; Soil Navigator Decision Support System, DSS; Debeljak et al., 2019). In addition, the assessment of different functions is partly based on the same information (Zwetsloot et al., 2021); e.g. SOM is a component in four out of five functions. How those pre-specified modelling relations affect the observed trade-offs and synergies among functions is unclear. While I think the efforts made using FLM (and the associated Soil Navigator Decision Support System, https://soilnavigator.eu/, last access: 20 May 2025) have great value for society in recommending changes based on the best knowledge today, I also believe we need to deepen our mechanistic understanding of the interrelations of the soil functioning and how they can be optimized. For this, I propose that we need a measurement and modelling framework that (1) allows quantitative assessment of soil functions based on independent data and (2) assesses functions and drivers at low spatial and temporal resolutions (Bradford et al., 2016, 2017; Fierer et al., 2021).
Many processes in soil depend on factors external to soil, such as temperature and water inputs. This contributes to the challenge in using many biological soil health indicators (Fierer et al., 2021) as they can become highly variable in time and space. To get around that, it was proposed that soils be incubated under standard conditions so that only factors internal to a soil would contribute to the observed functioning (Daou and Shipley, 2019). This is the approach I take here as well, and, as such, the proposed measurement system is focussed on estimating potential soil functioning and multifunctionality under a set of soil-external conditions optimal for plant growth. Below, I provide suggestions on how to link these measures to actual in situ rates of soil functioning. Nevertheless, I think this focus on the intrinsic – although not time-invariant – potential soil functioning is important as it can give the method predictive value for expected in situ soil functioning irrespective of the weather conditions that materialize during the growing season.
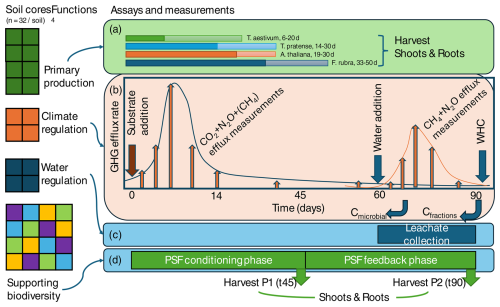
Here, I outline a proposal for a standardized soil multifunctionality assay that addresses the key soil functions in the functional land management framework (Schulte et al., 2014). The method is based on incubations of intact soil cores, subjected to several treatments, and measuring responses that are indicative of the underlying soil functions (Fig. 3; Table 1). The methods assume that all soils are sampled in the same way and incubated under standardized conditions, including temperature, light, watering regime, and air humidity, to ensure comparability (see Table 2 for a proposal). The goal is to estimate the intrinsic capacity of each soil for performing each soil function.
A soil sampling team will collect 32 soil monoliths (60 mm × 25 cm deep, ∼ 22.6 L soil) per soil. The monoliths are used to quantify primary production (a – eight green monoliths, two per bio-assay plant species), climate regulation (b – four orange monoliths, one for each substrate addition treatment), and water regulation (c – four blue monoliths for water storage and purification measurements) and for supporting plant biodiversity (d – 16 coloured monoliths, with each colour representing an indicator plant; this is the same as in (a), for which direct and indirect plant–soil feedback (PSF) is estimated in phase 2 (P2) in each of the four soils conditioned during phase 1 (P1)). The monoliths are incubated for 90 d under standard incubation conditions (Table 2). As such, the measurements target the capacity of a soil to deliver key soil functions under optimal conditions for plant growth. For both primary production and biodiversity functions, plant harvest days are fixed (indicated in days after the species name) and based on plant dry mass. Likewise, upon substrate addition (t0), gaseous efflux of CO2, N2O, and CH4 are measured on fixed days, with intensive sampling in the first 14 d and then less frequent sampling until day 90. In addition, microbial C and C in soil fractions (aggregates) are measured after 70 and 90 d. The water regulation measurements can be done independently in this setup and can potentially be shifted in time but are now placed at the end of the 90 d period to spread the workload over time. However, infiltration and leaching measurements will be conducted over a fixed time period.
Table 1Proposed approach to standardized quantification of the multiple functions of soils. The proposed indicators for each (sub-)function are used to fit an LVM that approximates the generalized soil function. RGR denotes relative growth rate (g g−1 d−1), and C N is the carbon nitrogen ratio.
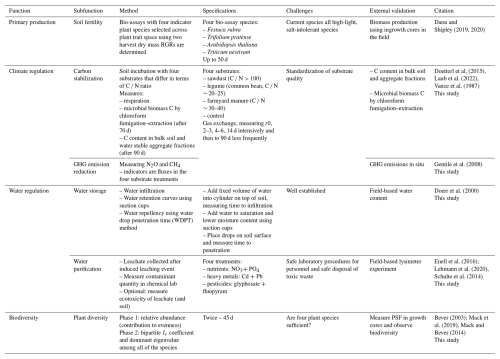
4.1 Primary production
For primary production, I follow the method developed by Daou and Shipley (2019), with which they assessed generalized soil fertility. They used four plant species as standard bio-assay indicators that span a wide range of ecological life history strategies (Table S1 in the Supplement). Using intact soil cores incubated under fixed environmental conditions in a growth cabinet, they estimated the relative growth rates (RGRs) of each of the species in each soil. They used that information to fit a measurement model, a specific type of latent-variable model, which estimates the values of the latent variable of generalized soil fertility (FG). The measurement model can be thought of as a kind of principal components analysis but with more constraints imposed on the solution; e.g. there is one common axis that all four indicator species map onto. The aforementioned authors have applied their method successfully to Canadian and French soils with herbaceous plant communities (Daou et al., 2021; Daou and Shipley, 2019), showing that their FG metric outperforms other metrics as predictors of primary plant production in mixed communities. With the help of Judith Nugteren (then intern at NIOO-KNAW), we applied their method to Dutch grassland soils, and our analysis confirms key aspects of their method (see the Supplement). We found that soils expected to be more fertile based on prior knowledge score higher on the generalized fertility index (FG), and the scores are on the same numerical scale as those of Daou and Shipley (2019); the fertility score is sensitive to fertilizer treatments (Hoagland solution), and replicate soil samples give similar scores, indicating a good level of repeatability.
To be representative of generalized soil fertility and, thus, primary productivity, the indicator species have to be as ecologically different as possible in order to capture the maximum diversity in responses while being able to grow them together in the same abiotic conditions (light, temperature, soil water levels). Daou and Shipley used herbaceous species of open grassland habitats and chose phytometer species that (1) were as different as possible according to their ecology and taxonomy; (2) have seeds that are easy to acquire by researchers worldwide; and (3) have seeds from recognizable, reproducible, and stabilized varieties. The selected species (Table S1) cover an interesting gradient of plants, with different root-associated mutualists, growth rates, and lifespans. However, all of them require high light, are salt intolerant, and do not reflect extreme soil acidities (Lamontagne and Shipley, 2022). The question is, thus, if, indeed, these four species are the optimal ones to select when used in an integrated assessment of soil multifunctionality aiming to be applied worldwide.
4.2 Climate regulation
Climate regulation as a soil function has to be split into two subfunctions (Table 1) due to the large differences in soil processes involved: on the one hand, carbon storage and, on the other hand, preventing emissions of other greenhouse gases (mainly N2O and CH4; Van de Broek et al., 2019). Carbon is stabilized long term in the soil when it is fixed to a mineral particle matrix or bound in aggregates by microbes (Cotrufo et al., 2019; Lavallee et al., 2020; Lehmann and Kleber, 2015). This happens through microbial biochemical transformations of rhizodeposits, litter, and microbial necromass (Kou et al., 2023; Sokol et al., 2022). The extent to which this happens depends on the physico-chemical quality of substrate inputs and the soil matrix properties (Georgiou et al., 2022). Nitrous oxide emissions mainly result from microbial transformations of fertilizers containing reactive nitrogen (Tian et al., 2020; Van de Broek et al., 2019; Zhou et al., 2017), while methane emissions mainly occur under anaerobic conditions when soils are waterlogged and methanogen activity is high (Dalal and Allen, 2008; Levy et al., 2012). However, soils can also be sinks of methane and nitrous oxide through methanotrophy and nitrous oxide consumption (Dutaur and Verchot, 2007; Gatica et al., 2020; Tian et al., 2020).
I think we can estimate both subfunctions using a single incubation setup (Table 1, Fig. 3), where we use substrate additions to elicit soil responses. We can estimate carbon stabilization and, thus, storage capacity by incubating a set of four standard substrates that vary widely in their biogeochemical quality. High N substrates will also induce N2O efflux. From low to high quality, I propose using sawdust (C N > 100), farmyard manure (FYM; C N ∼ 30–40), common bean (Phaseolus vulgaris, C N ∼ 20–25), and a control where nothing is added (only basal respiration). Upon substrate addition, the soils will be incubated at the same conditions as above (Table 2), and gas efflux will be regularly sampled for ∼ 90 d, with intensive sampling for the first 14 d. Using a gas chromatograph also suitable for quantifying CO2, N2O, and CH4, all three major greenhouse gases could be monitored simultaneously. Since CO2 efflux may not reflect the longer-term C fate, I also propose measuring soil microbial C using chloroform fumigation–extraction (Vance et al., 1987) and the C content of soil fractions (bulk soil, large macroaggregates (LMAs, > 2 mm), small macroaggregates (SMAs, 2–0.25 mm), micro-aggregates (MiAs, 0.25–0.053 mm), and free particles of the silt and clay fraction (SiCl, < 0.053 mm) not included in aggregates (Laub et al., 2022; Six et al., 2000). Microbial C and C in soil fractions will be determined for samples taken on day 70 and 90, respectively (Laub et al., 2022), and will be analysed using a CN analyser. For substantial CH4 production to occur, anaerobic conditions are needed, and so sampling for CH4 efflux will need to be combined with the water storage measurements where soil cores are wetted till saturation.
A key challenge here is how to standardize the substrates. The best way would be to implement a standard protocol to purposely cultivate the needed substrates directly, e.g. growing common bean in potting soil under standard conditions, harvesting, drying, and applying on a mass basis. For sawdust and farmyard manure, this is less straightforward. Instead of FYM, compost may be an alternative; however, for both, nutrient content varies among suppliers. Alternatively, a set of synthetically produced compounds varying in terms of their C N ratio could be used to better standardize the substrate input, but they need to have sufficient complexity to reflect real-world conditions.
4.3 Water regulation
Water regulation has been defined as “the capacity of the soil to remove harmful compounds and the capacity of the soil to receive, store, and conduct water for subsequent use and to prevent droughts, flooding, and erosion” (Wall et al., 2020). Water storage is the result of a balance between infiltration and runoff during precipitation events, holding water in the soil matrix, and losses to evapotranspiration and percolation to deeper soil layers and aquifers. Water purification is concentrated on the breakdown and sequestration of harmful compounds (Keesstra et al., 2012; Wall et al., 2020).
For water storage capacity, I propose measuring infiltration rate and water repellence (hydrophobicity) and estimating the water retention curve, including water-holding capacity. Infiltration is the key input for water in most systems, but a lack of infiltration may also importantly impact soil functioning by generating horizontal soil runoff and erosion and, alternatively, by waterlogging. To capture these elements a substantial water influx needs to be tested. Water repellence can easily be tested using the water drop penetration time (WDPT) method (Doerr et al., 2000) and reflects an important soil property when it is extremely dry or upon burning, preventing infiltration (Stoof et al., 2011). The water retention curve can be estimated using standard protocols (see, for example, ISO 11274:2019, https://www.iso.org/standard/68256.html, last access: 20 May 2025), e.g. estimating parameters of the non-linear van Genuchten model. Based on the retention curves, estimated values for field capacity (−33 kPa) and permanent wilting point Pw (−1500 kPa) will be used in the fitting of a latent-variable model for water storage capacity.
With respect to purification (natural attenuation), the EU Water Framework Directive focusses on nutrients, pesticides, and trace elements for groundwater-mediated contamination (European Parliament and the Council, 2006). Following Lehmann et al. (2020) and Wall et al. (2020), I propose measuring NO3 (Nolan and Stoner, 2000), NH4, and P in the leachate collected after applying a standardized amount of polluted water to the soil core to estimate nutrient retention capacity. The scale used will be the percentage recovery of the introduced amount of each nutrient upon measurement using a continuous flow analysis AutoAnalyzer. For purification and retention of pesticides (Froger et al., 2023; Tang and Maggi, 2021), water polluted with glyphosate and fluopyram will be added to the soil cores and concentrations measured in the collected leachate. Glyphosate (https://sitem.herts.ac.uk/aeru/ppdb/en/Reports/373.htm, last access: 20 May 2025) is a commonly used herbicide. It is the most leached pesticide globally (Tang and Maggi, 2021) and was dominantly found in a French national survey (Froger et al., 2023) despite being characterized as relatively immobile and of low leachability in soils. It is moderately toxic to earthworms, fish, crustaceans, and birds but is still approved for used in the EU. Also, its major biodegradation product, aminomethylphosphonic (AMPA), needs to be quantified as it is also toxic to earthworms. Fluopyram (https://sitem.herts.ac.uk/aeru/ppdb/en/Reports/1362.htm, last access: 20 May 2025) is a fungicide with nematicidal side effects and is highly leachable and moderately toxic to aquatic life and earthworms. It is approved in the EU and was frequently found in France (Froger et al., 2023). Both pesticides can be quantified using reversed-phase high-performance liquid chromatography coupled to a quadrupole mass spectrometer (HPLC-MS/MS; Froger et al., 2023). To estimate heavy-metal retention I propose measuring Pb and Cd concentrations in leachate collected upon the application of standardized polluted water to the soil cores. These two elements can be used to predict cation heavy-metal behaviour, known to negatively affect soil organisms and plants (Nagajyoti et al., 2010; de Vries et al., 2007) in general. Both can be estimated using flame atomic absorption spectrometry (FAAS; America Public Health Association, 2017). The required input concentrations of the pollutants for sensitive indicator use need to be derived empirically.
While I think the response quantification (the indicators) should best be done by assessment of the chemical concentrations in the leachate, this can be expensive and unfeasible for less resource-rich labs. As an alternative, I propose conducting bio-assays on aquatic life. For instance, algal growth can be used to quantify responses to nutrient leaching, and ecotoxicology protocols (e.g. using Daphnia spp.) can be used to assess the toxic potential of the soil leachate. I think the nutrient leachate needs to contain all assessed nutrients in combination to avoid specific nutrient limitations for the algae. For the toxicity tests, each compound (heavy metal, pesticide) needs to be tested separately to estimate their pure impact. However, it is known that mixtures are most toxic for soil biodiversity (Beaumelle et al., 2023), and so a treatment where aliquots of each contaminant are mixed may be critical for extrapolation to field conditions. Furthermore, how direct chemical quantification and ecotoxicology tests need to be compared across studies requires further study. Likewise, it is an open question as to whether responses to such different chemicals can be captured effectively by a single latent variable. Luckily, measurement model evaluation procedures will quickly inform the researcher if a further division into subfunctions is needed.
The impacts of leached contaminants also depend on the subsoil characteristics (Brookfield et al., 2021), and so the topsoil flux estimated here does not inform us regarding the whole impact of a soil on its aqueous surroundings. Indeed, models are needed that predict the fate of such leached contaminants in a given soil and landscape. Luckily, subsoils are primarily governed by abiotic properties and processes, less so by biological processes, and modelling could thus be more straightforward.
4.4 Supporting biodiversity
For biodiversity, I focus on a soil's potential for supporting plant diversity. Plant diversity within a given location, on the scale of the interacting plants (Casper et al., 2003), is maintained by preventing or delaying competitive exclusion (Fukami and Nakajima, 2013; Hardin, 1960). In most terrestrial communities, this is importantly mediated by soil-borne antagonists (Bever et al., 2015; Mack et al., 2019), the net effects of which can be quantified by measuring the soil's plant–soil feedback (Bever, 2003; Van der Putten et al., 2013).
Plant–soil feedback (PSF) is typically measured using a two-phase greenhouse experiment. In the first phase, plants are grown to condition the soil; i.e. they change the soil community and abiotic conditions in their species-specific way (Van der Putten et al., 2013). In particular, they increase the abundance of their associated soil-borne antagonists and mutualists. In the second or feedback phase, individuals from the same species or a different species are grown in the soil, and the difference in biomass they produce across differently conditioned soils provides information on the net plant–soil feedback. Such data can be used to predict the long-term coexistence of species using relatively simple mathematical models that have recently been extended from pairwise to multispecies models (Bever et al., 1997; Mack et al., 2019). These models can be parameterized by measuring PSF in a full-factorial soil conditioning and feedback design. Here, I propose implementing such a design for an artificial community of four plant species with two growth phases of 45 d each (Fig. 3). From the model we can estimate the net pairwise interaction coefficient (Is) among the species pairs but also the real part of the dominant eigenvalue among all of the species, which is a predictive measure for coexistence and stability in the face of local species extinctions (Mack et al., 2019).
4.5 A new measurement framework for soil multifunctionality
Once the selected indicators of the multiple soil functions have been measured under standardized conditions for a range of soils, we can start evaluating the adequacy of the latent-variable model for each function. Measurement models for the soil function latent variables can be fit using standard tools used in the social sciences under the term factor analysis (Grace, 2006; Shipley, 2016); this includes ML-based estimation in R package lavaan (Rosseel, 2012). Model fit should first be assessed for the component measurement models.
One of the key steps to ensure comparability across labs will be to use internal benchmarks. Benchmarks are used for temperature – for instance, by fixing the high and low end of the scale to the boiling and freezing point of water, respectively. We can do the same for soil functioning. For instance, for primary production, I propose using pure bare sand (e.g. standard sand used for testing cement; ISO 679:2009(en); https://www.iso.org/standard/45568.html, last access: 20 May 2025) for the low end of the scale, while high-quality potting soil (growing medium) can be used for the high end of the scale (Fig. 4). I also predict that the subfunctions of water storage and purification and carbon storage capacity will be meaningfully mapped using these two internal benchmarks. Whether biodiversity regulation also maps to these two extremes needs to be explored.
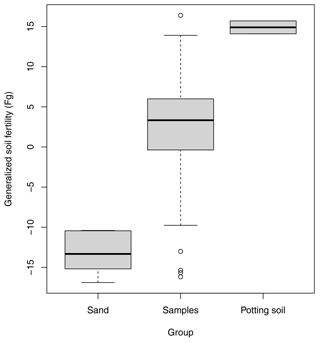
Figure 4Use of internal controls to benchmark the estimated latent variables representing soil functions. Here, potting soil and poor sand were used to benchmark the high and low ends of the generalized soil fertility scale, respectively. The samples included 30 soils selected from within the Netherlands with contrasting fertility. Soil samples were taken as field homogenates and incubated in a greenhouse for 50 d. Unless explicitly stated otherwise, Judith Nugteren and I followed the procedures of Daou and Shipley (2019). Four indicator plant species were grown in separate pots for each soil and harvested, dried, and weighed at two points in time per plant species. From these data, the relative growth rates per species and soil were estimated and used to fit a measurement model from which a single latent variable was extracted, called generalized soil fertility (FG). See the Supplement for a detailed protocol, results, and a discussion.
Another key step will be external validation of the proposed soil function measurement instruments. For this, we can leverage long-term established field experiments and research networks such as the ILTER sites for arable systems (Trajanov et al., 2019) and the Nutrient Network for (semi-)natural grasslands (Borer et al., 2017). Within these networks important soil functions are measured, often over time, and provide a good context for comparing the ex situ soil function assessments, quantifying soil potential for soil functioning, proposed here with actual in situ measurements. I wonder to what extend the same approaches as those I work out here (Sect. 4) can be used to assess in situ soil functioning as well. Primary productivity and biodiversity regulation can be tested in the field by using in-growth cores in the field directly or by using camera systems (rhizotrons; Downie et al., 2015). Lysimeters can be installed to assess leachate contaminations, and GHG emissions can be measured in response to substrate additions. This would allow for an explicit 1:1 linkage with the ex situ soil potential measurements, allowing for cross-global comparability, and in-situ measurements that estimate real-world soil performance. This crucial step can help us build up the causal machinery to link soil intrinsic and extrinsic factors together in a common model to explain and predict soil multifunctionality and, thus, soil health in reality.
With this proposal to measure the four key soil functions in hand, we can put the assessment of soil multifunctionality on a common foundation. Naturally, this is an initial proposal, and, through discussion and collaboration, I think it will need to be refined (see Sect. 5 for several key concerns and points of improvement). In Fig. 3, there is a schematic representation of the experimental setup needed to implement the proposed scheme. The whole process involves taking 32 soil cores per target soil and incubating them together for 90 d and taking various samples and measurements in the meantime. The setup replies on simple equipment as much as possible. However, critical infrastructure is the incubation facility, e.g. controlled growth cabinets or greenhouses. In addition, a gas chromatograph, CN analyser, AutoAnalyzer, HPLC, and FAAS are needed. For labs without access to this high-end equipment, collaborations with larger labs need to be set up to conduct these analyses. For pollutants, using ecotoxicology approaches represents a low-cost alternative, but that needs to be calibrated to the analytical chemistry data. It will be clear that the setup is not feasible for regular soil testing for commercial services, given the long-term incubation period, but that is not the intent.
Generally, the practical and logistical choices for method selection in soil quality assessments vary depending on the objectives: mechanistic understanding, functional land management, and/or large-spatial-scale monitoring (Creamer et al., 2022). However, the scheme I propose here aims to strike a balance between these three objectives. The use of measurement models linked to functions important to land management, standardized measurements that can be compared across labs and thus potentially scaled up, and a flexible framework that allows the integrated study of underlying mechanisms make this three-way integration possible. The question is this: how well will it do all three?
Currently, I propose that samples be collected as intact soil cores to preserve soil structure and macroscopic features of soil so that the real vertical and horizontal variations are reflected in the measurements. These replicate cores need to come from small homogeneous areas, accounting for variations in microclimate, soil type, and land use and management. However, it was shown that intact cores and homogenized soils generate almost identical pictures of soil fertility (Daou and Shipley, 2020), which would make for much easier sample collection and handling. Similarly, earlier studies using substrate additions sometimes incubate as little as 80 g of soil (Doetterl et al., 2015); this would strongly minimize substrate and soil requirements and may be an improvement over what I propose here. Likewise, Daou and Shipley (2019) conduct their work in a highly controlled growth cabinet, but could the data still be measured with acceptable error variances in a glasshouse, a screenhouse, or a common garden setup? In a common garden, of course, temperature and light cannot be controlled, but maybe their impact can effectively be approximated by using growing degree days as measured by a local weather station or temperature loggers?
Loosening up this constraint will be important for application of the method in the Global South where high-tech facilities are strongly limiting. In general, the approach may be challenging to implement “as is” in the Global South and potentially in other labs as well. Currently, the method relies on some advanced lab analytical equipment to get all the required measurements. Further work needs to focus on gaining meaningful measurements using simpler approaches, but they need to be validated against the robust methods identified here.
What about sampling time? Do we need to include seasonal dynamics, e.g. reflecting the massive turnover of bacterial and fungal communities over the year (Schadt et al., 2003), or can we select a single most predictive period? I think it would be most valuable if we could sample in the seasonally cold and/or dry period when plant growth is most limited. Then we could compare in situ soil functioning data in the field during the subsequent growing season to our prior off-season ex situ estimates. These linkages could be used to build predictive models. An alternative would be to sample during the peak season, but then, often, (1) farmers are busy on their field, (2) crops are damaged by sampling and walking, and (3) researchers are occupied with other field experiments and observations.
Here, I propose incubating soils under standard soil-external conditions optimal for plant growth (see Table 2). However, can these conditions be applied to all soils? What about soils that experience regular waterlogging? What about soils from low- or high-temperature conditions: will the shift to mesic conditions cause unnatural behaviour of these soils? Can we shorten the protocol? For biodiversity regulation, I propose conducting two-phase plant–soil feedback experiments (Bever, 1994; Van der Putten et al., 2013), but from the first phase alone, we can also use the shoot biomass data to get an initial idea of the soil's ability to support plant diversity by looking at the evenness of the relative abundances (Pielou, 1966). Could that be predictive of phase-2 competitive hierarchies?
I am strongly in favour of reporting on the measured soil functions separately so that fellow scientists, policymakers, and the public can make their own assessment and overlay their own priorities with respect to the multiple functions of soil. However, can these measure not be combined into a single indicator? If they are combined with reports of the individual functions, I think they can be. There is a huge body of literature on multi-objective optimization methods (Pereira et al., 2022) where combining objectives is operationalized using explicit rules and criteria. Such optimization should be done with maximum transparency about how functions are weighted and combined for the aggregate index to have any practical use. Also, the weighing should be informed by involving multiple stakeholder group consultations, e.g. using focus group discussions (Bampa et al., 2019; Schulte et al., 2019).
The methods I propose are too cumbersome to be used directly in commercial soil testing but are crucial to advance our foundational understanding. In order to be useful, indicators need to be conceptually relevant; sensitive to changes; informative for management; and effective, e.g. cheap and fast (Lehmann et al., 2020). I argue that my method is conceptually relevant and sensitive and that, when the measurements are explicitly linked to environmental and management data, the results can be used to inform management decisions. The effectiveness is something requiring further testing; see the preceding discussion for steps I want to take. Additionally, we should explore how these soil functioning measurements can be approximated by high-throughput screening techniques such as near-infrared spectroscopy, X-ray fluorescence, and potentially eco-acoustics and environmental DNA.
Finally, to scale up and inform spatial planning and management choices worldwide, the measurements need to be integrated into a strong framework, explaining the potential, the synergies, and the trade-offs among functions mechanistically (Fierer et al., 2021). Including biology in these models is key (Creamer et al., 2022; Fierer et al., 2021). As recently as 2004, a map of known soil threats and degradations published by Science listed only physical and chemical forms of soil degradation and was solely focussed on agricultural production (AAAS, 2004). We have moved on but into unknown territory. The mechanistic machinery is, for an important part, there in the literature but needs to be conceptually brought together, e.g. by using plant–microbe–soil stoichiometry as an organizing principle.
In the wake of the Green Revolution, seeing widespread application of chemical fertilizers and pesticide control, the importance of soil science has dwindled. Now, due to the threats exerted on human societies by climate change and biodiversity loss, soil has been revalued as a central nexus integrating many aspects of human wellbeing (Sigl et al., 2023). I believe that the study of soil multifunctionality and, thus, soil health should lie at the heart of this new valuation of soil and soil biodiversity and should be a key focus area in order to bring humanity within the planetary boundaries (Steffen et al., 2015) while simultaneously developing sustainable livelihoods for all (Dearing et al., 2014; Fanning et al., 2022). This also means that we have to put the study of soil multifunctionality on solid empirical and theoretical footing, for which this paper develops a concrete proposal (Sect. 4; Fig. 3).
A key improvement is that I separated the causes and consequences of the soil functions. Focussing on the consequences allows standardized measurements that can be adopted across laboratories, both foundational and applied research oriented, and allows them to be linked flexibly, via the estimated latent variables, to competing mechanistic frameworks through structural equation models. Linking the ex situ functional measurements by mechanistic causal models is also important to understand the results within their environmental context. It is well known that soil health indicators need to be interpreted in site-specific ways (Creamer et al., 2022; Vogel et al., 2018), and that means that a global understanding needs to account for the relevant site specificities. For instance, clay content determines what range of values to expect for organic matter content (Lehmann et al., 2020), while soil texture shapes ecosystem recovery trajectories (Bach et al., 2010). A key question will be “how unique are the properties and functions in this soil?” compared to the soils in our reference set. To what extent can we extrapolate our results meaningfully and based on which (minimum) set of parameters? To answer these questions we need to bring soil functional and contextual measurements together in a common global database.
6.1 Outlook
There is a strong need to adjust our spatial planning of land use to best fit to the natural capabilities of soils, for which we need to know which soils do what functions best (Lehmann and Stahr, 2010). In addition, for optimal management, we need to know which functions can be combined for any given soil and at what level of performance. When both of these aspects are combined we can perform spatial optimization where the service delivery capacity of our soils is explicitly linked to the service provision required by society, e.g. under different climate and socio-economic scenarios (Pereira et al., 2010). In this way, we can also get beyond the challenge of different valuation of functions by individual stakeholders (Allan et al., 2015; Lehmann et al., 2020; Manning et al., 2018) by organizing around societal needs in aggregate.
Here, I limited the soil function set to the four key functions from the land management framework (Debeljak et al., 2019; Schulte et al., 2014; Zwetsloot et al., 2021); however, soils are involved in more functions so should we expand the set? What about the quality of the plants produced? Could we measure tissue N and vitamin content to indicate food and feed quality? What about direct and indirect contributions to human health (Sun et al., 2023; Wall et al., 2015)? Can the soil suppress zoonoses and human disease agents? Does a well-managed soil strengthen the human-associated microbiome and immune systems? Does it reduce allergies? Is it a better source of therapeutics (Thiele-Bruhn, 2021)? What about crop-associated disease suppression (Sagova-Mareckova et al., 2022)? To some extent, this will be reflected in the primary production and biodiversity functions, but disease agents are often host specific. How can we generate an overall picture of the general and specific disease suppressiveness of a given soil? Can this be done only through sequencing or can bio-assays of representative pathogens reflect the activity of broad suites of organisms? And what about habitats for soil life or the larval stages of aboveground arthropods? Can we find four indicator species to derive simple tests, such as for plant diversity? Do we need eDNA sequencing to predict belowground diversity and composition? What about the predictive capabilities of these measurements? How quickly does their predictive capacity decline over time (Petchey et al., 2015)? What about resistance and resilience to disturbance? Should experimental treatments be included in the setup (Harris et al., 2022)? I suppose an additional period of tier-2 testing can be implemented once the main measurements have been taken.
Here, I have worked out a simple but causally consistent methodology to quantify soil multifunctionality and, thus, soil health. The system is based on latent-variable modelling (LVM), with each LV capturing one crucial soil function: primary production, climate regulation (split into carbon storage and GHG emission reduction), water regulation (split into water storage and purification capacity), and biodiversity regulation (captured as plant diversity potential). This system makes explicit the fact that soil functions are complex soil properties, contingent on many drivers, that cannot be measured directly using any device. It also explicitly separates the causes and consequences of each soil function. Using the consequences as indicators, we can estimate the LV factors that approximate the soil intrinsic capacity to perform each function. For example, we can estimate soil fertility from plant growth. I hope this can be a common point of departure in the soil health field to allow scientists to band together and to organize soil multifunctionality and soil health research more mechanistically.
The R code to fit the soil multifunctionality measurement models and to analyse the Dutch generalized soil fertility model is available on GitHub at https://github.com/JasperWubs/SoilMFv0.1 and https://doi.org/10.5281/zenodo.16946924 (Wubs, 2025, last access: 26 August 2025). This also includes code simulating Simpson's paradox.
The data for the generalized soil fertility test in Dutch soils are available as supplementary data at https://doi.org/10.6084/m9.figshare.29132744 (Wubs and Nugteren, 2025, last access: 26 August 2025).
The supplement related to this article is available online at https://doi.org/10.5194/soil-11-609-2025-supplement.
I developed the concept from the earlier work of Laurent Daou and Bill Shipley. I worked out the measurement framework and led the Dutch generalized soil fertility index experiment and analysed the data. I wrote the paper.
The author has declared that there are no competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
I dedicate this paper to Sewall Wright FRS for having invented path analysis and for the difficulties he experienced in having his method accepted. This paper is the result of many interactions with colleagues, for which I am very grateful. In particular, I want to thank Bill Shipley (University of Sherbrooke) for introducing me to the concept of measurement models during his Wageningen structural equation modelling course. I want to thank Johan Six (ETH Zürich) and Paul Bodelier (NIOO-KNAW) for their thoughts on measuring soil carbon storage and greenhouse gas emissions from soils. Walter Schenkeveld (WUR), Bert-Jan Groenenberg (WUR), and Michiel Rutgers (RIVM) helped with the discussions on measuring the soil's capacity for purification of pollutants. Thanks also to Ciska Veen, Wim van der Putten, and Merlijn Schram (all NIOO-KNAW), who provided general reflections on quantifying soil multifunctionality and framing the story. Judith Nugteren (then HAS Green Academy) helped me apply the generalized soil fertility index and some extensions to Dutch soils (Fig. 4) – thanks are given for her enthusiasm and diligent work. Finally, I gratefully thank my partner, Ruth van Werven, and my family for all of their efforts to support me during good and bad times.
This research was funded by the European Union (MSCA Postdoctoral Fellowship, MultiSol project, grant no. 101066007 to E. R. Jasper Wubs). The views and opinions expressed in this paper are, however, those of the author only and do not necessarily reflect those of the European Union or the European Research Executive Agency (REA). Neither the European Union nor the granting authority can be held responsible for them. The granting authority had no influence over the content of the work.
This paper was edited by Luis Merino-Martín and reviewed by Julien Demenois and one anonymous referee.
AAAS: Soil and Trouble, Science, 304, 1614–1615, https://doi.org/10.1126/science.304.5677.1614, 2004.
Allan, E., Manning, P., Alt, F., Binkenstein, J., Blaser, S., Blüthgen, N., Böhm, S., Grassein, F., Hölzel, N., Klaus, V. H., Kleinebecker, T., Morris, E. K., Oelmann, Y., Prati, D., Renner, S. C., Rillig, M. C., Schaefer, M., Schloter, M., Schmitt, B., Schöning, I., Schrumpf, M., Solly, E., Sorkau, E., Steckel, J., Steffen‐Dewenter, I., Stempfhuber, B., Tschapka, M., Weiner, C. N., Weisser, W. W., Werner, M., Westphal, C., Wilcke, W., and Fischer, M.: Land use intensification alters ecosystem multifunctionality via loss of biodiversity and changes to functional composition, Ecol. Lett., 18, 834–843, https://doi.org/10.1111/ele.12469, 2015.
America Public Health Association: 3111 metals by flame atomic absorption spectrometry, in: Standard Methods For the Examination of Water and Wastewater, American Public Health Association, Washington DC, USA, https://doi.org/10.2105/SMWW.2882.043, 2017.
Bach, E. M., Baer, S. G., Meyer, C. K., and Six, J.: Soil texture affects soil microbial and structural recovery during grassland restoration, Soil Biol. Biochem., 42, 2182–2191, https://doi.org/10.1016/j.soilbio.2010.08.014, 2010.
Bampa, F., O'Sullivan, L., Madena, K., Sandén, T., Spiegel, H., Henriksen, C. B., Ghaley, B. B., Jones, A., Staes, J., Sturel, S., Trajanov, A., Creamer, R. E., and Debeljak, M.: Harvesting European knowledge on soil functions and land management using multi-criteria decision analysis, Soil Use Manag., 35, 6–20, https://doi.org/10.1111/sum.12506, 2019.
Baveye, P. C., Baveye, J., and Gowdy, J.: Soil “Ecosystem” Services and Natural Capital: Critical Appraisal of Research on Uncertain Ground, Front. Environ. Sci., 4, https://doi.org/10.3389/fenvs.2016.00041, 2016.
Beaumelle, L., Tison, L., Eisenhauer, N., Hines, J., Malladi, S., Pelosi, C., Thouvenot, L., and Phillips, H. R. P.: Pesticide effects on soil fauna communities – A meta-analysis, J. Appl. Ecol., 60, 1239–1253, https://doi.org/10.1111/1365-2664.14437, 2023.
Bever, J. D.: Feedback between plants and their soil communities in an old field community, Ecology, 75, 1965–1977, https://doi.org/10.2307/1941601, 1994.
Bever, J. D.: Soil community feedback and the coexistence of competitors: conceptual frameworks and empirical tests, New Phytol., 157, 465–473, https://doi.org/10.1046/j.1469-8137.2003.00714.x, 2003.
Bever, J. D., Westover, K. M., and Antonovics, J.: Incorporating the soil community into plant population dynamics: the utility of the feedback approach, J. Ecol., 85, 561–573, 1997.
Bever, J. D., Mangan, S., and Alexander, H. M.: Maintenance of plant species diversity by pathogens, Annu. Rev. Ecol. Evol. Syst., 46, 305–325, https://doi.org/10.1146/annurev-ecolsys-112414-054306, 2015.
Blackwell, M. S. A., Darch, T., and Haslam, R. P.: Phosphorus Use Efficiency and Fertilizers: future opportunities for improvements, Front. Agric. Sci. Eng. – FASE, 6, 332–340, https://doi.org/10.15302/J-FASE-2019274, 2019.
Borer, E. T., Grace, J. B., Harpole, W. S., MacDougall, A. S., and Seabloom, E. W.: A decade of insights into grassland ecosystem responses to global environmental change, Nat. Ecol. Evol., 1, 0118, https://doi.org/10.1038/s41559-017-0118, 2017.
Bradford, M. A., Wood, S. A., Bardgett, R. D., Black, H. I. J., Bonkowski, M., Eggers, T., Grayston, S. J., Kandeler, E., Manning, P., Setälä, H., and Jones, T. H.: Reply to Byrnes et al.: Aggregation can obscure understanding of ecosystem multifunctionality, P. Natl. Acad. Sci. USA, 111, E5491–E5491, https://doi.org/10.1073/pnas.1421203112, 2014.
Bradford, M. A., Berg, B., Maynard, D. S., Wieder, W. R., and Wood, S. A.: Understanding the dominant controls on litter decomposition, J. Ecol., 104, 229–238, https://doi.org/10.1111/1365-2745.12507, 2016.
Bradford, M. A., Veen, G. F., Bonis, A., Bradford, E. M., Classen, A. T., Cornelissen, J. H. C., Crowther, T. W., Long, J. R. D., Freschet, G. T., Kardol, P., Manrubia-Freixa, M., Maynard, D. S., Newman, G. S., Logtestijn, R. S. P., Viketoft, M., Wardle, D. A., Wieder, W. R., Wood, S. A., and van der Putten, W. H.: A test of the hierarchical model of litter decomposition, Nat. Ecol. Evol., 1, 1836–1845, https://doi.org/10.1038/s41559-017-0367-4, 2017.
Brookfield, A. E., Hansen, A. T., Sullivan, P. L., Czuba, J. A., Kirk, M. F., Li, L., Newcomer, M. E., and Wilkinson, G.: Predicting algal blooms: Are we overlooking groundwater?, Sci. Total Environ., 769, 144442, https://doi.org/10.1016/j.scitotenv.2020.144442, 2021.
Bünemann, E. K., Bongiorno, G., Bai, Z., Creamer, R. E., De Deyn, G., de Goede, R., Fleskens, L., Geissen, V., Kuyper, T. W., Mäder, P., Pulleman, M., Sukkel, W., van Groenigen, J. W., and Brussaard, L.: Soil quality – A critical review, Soil Biol. Biochem., 120, 105–125, https://doi.org/10.1016/j.soilbio.2018.01.030, 2018.
Byrnes, J. E. K., Gamfeldt, L., Isbell, F., Lefcheck, J. S., Griffin, J. N., Hector, A., Cardinale, B. J., Hooper, D. U., Dee, L. E., and Duffy, J. E.: Investigating the relationship between biodiversity and ecosystem multifunctionality: challenges and solutions, Methods Ecol. Evol., 5, 111–124, https://doi.org/10.1111/2041-210X.12143, 2014.
Byrnes, J. E. K., Roger, F., and Bagchi, R.: Understandable multifunctionality measures using Hill numbers, Oikos, 2023, e09402, https://doi.org/10.1111/oik.09402, 2023.
Casper, B. B., Schenk, H. J., and Jackson, R. B.: Defining a plant's belowground zone of influence, Ecology, 84, 2313–2321, https://doi.org/10.1890/02-0287, 2003.
Cotrufo, M. F., Ranalli, M. G., Haddix, M. L., Six, J., and Lugato, E.: Soil carbon storage informed by particulate and mineral-associated organic matter, Nat. Geosci., 12, 989–994, https://doi.org/10.1038/s41561-019-0484-6, 2019.
Creamer, R. E., Barel, J. M., Bongiorno, G., and Zwetsloot, M. J.: The life of soils: Integrating the who and how of multifunctionality, Soil Biol. Biochem., 166, 108561, https://doi.org/10.1016/j.soilbio.2022.108561, 2022.
Dalal, R. C. and Allen, D. E.: Greenhouse gas fluxes from natural ecosystems, Aust. J. Bot., 56, 369–407, https://doi.org/10.1071/BT07128, 2008.
Daou, L. and Shipley, B.: The measurement and quantification of generalized gradients of soil fertility relevant to plant community ecology, Ecology, 100, e02549, https://doi.org/10.1002/ecy.2549, 2019.
Daou, L. and Shipley, B.: Simplifying the protocol for the quantification of generalized soil fertility gradients in grassland community ecology, Plant Soil, 457, 457–468, https://doi.org/10.1007/s11104-020-04729-4, 2020.
Daou, L., Garnier, É., and Shipley, B.: Quantifying the relationship linking the community-weighted means of plant traits and soil fertility, Ecology, 102, e03454, https://doi.org/10.1002/ecy.3454, 2021.
Dearing, J. A., Wang, R., Zhang, K., Dyke, J. G., Haberl, H., Hossain, Md. S., Langdon, P. G., Lenton, T. M., Raworth, K., Brown, S., Carstensen, J., Cole, M. J., Cornell, S. E., Dawson, T. P., Doncaster, C. P., Eigenbrod, F., Flörke, M., Jeffers, E., Mackay, A. W., Nykvist, B., and Poppy, G. M.: Safe and just operating spaces for regional social-ecological systems, Glob. Environ. Change, 28, 227–238, https://doi.org/10.1016/j.gloenvcha.2014.06.012, 2014.
Debeljak, M., Trajanov, A., Kuzmanovski, V., Schröder, J., Sandén, T., Spiegel, H., Wall, D. P., Van de Broek, M., Rutgers, M., Bampa, F., Creamer, R. E., and Henriksen, C. B.: A Field-Scale Decision Support System for Assessment and Management of Soil Functions, Front. Environ. Sci., 7, 115, https://doi.org/10.3389/fenvs.2019.00115, 2019.
Delgado-Baquerizo, M., Maestre, F. T., Reich, P. B., Jeffries, T. C., Gaitan, J. J., Encinar, D., Berdugo, M., Campbell, C. D., and Singh, B. K.: Microbial diversity drives multifunctionality in terrestrial ecosystems, Nat. Commun., 7, 10541, https://doi.org/10.1038/ncomms10541, 2016.
Doerr, S. H., Shakesby, R. A., and Walsh, R. P. D.: Soil water repellency: its causes, characteristics and hydro-geomorphological significance, Earth-Sci. Rev., 51, 33–65, https://doi.org/10.1016/S0012-8252(00)00011-8, 2000.
Doetterl, S., Stevens, A., Six, J., Merckx, R., Van Oost, K., Casanova Pinto, M., Casanova-Katny, A., Muñoz, C., Boudin, M., Zagal Venegas, E., and Boeckx, P.: Soil carbon storage controlled by interactions between geochemistry and climate, Nat. Geosci., 8, 780–783, https://doi.org/10.1038/ngeo2516, 2015.
Dooley, Á., Isbell, F., Kirwan, L., Connolly, J., Finn, J. A., and Brophy, C.: Testing the effects of diversity on ecosystem multifunctionality using a multivariate model, Ecol. Lett., 18, 1242–1251, https://doi.org/10.1111/ele.12504, 2015.
Downie, H. F., Adu, M. O., Schmidt, S., Otten, W., Dupuy, L. X., White, P. J., and Valentine, T. A.: Challenges and opportunities for quantifying roots and rhizosphere interactions through imaging and image analysis, Plant Cell Environ., 38, 1213–1232, https://doi.org/10.1111/pce.12448, 2015.
Dutaur, L. and Verchot, L. V.: A global inventory of the soil CH4 sink, Global Biogeochem. Cy., 21, GB4013, https://doi.org/10.1029/2006GB002734, 2007.
Enell, A., Lundstedt, S., Arp, H. P. H., Josefsson, S., Cornelissen, G., Wik, O., and Berggren Kleja, D.: Combining Leaching and Passive Sampling To Measure the Mobility and Distribution between Porewater, DOC, and Colloids of Native Oxy-PAHs, N-PACs, and PAHs in Historically Contaminated Soil, Environ. Sci. Technol., 50, 11797–11805, https://doi.org/10.1021/acs.est.6b02774, 2016.
European Parliament and the Council: Directive 2006/118/EC of the European Parliament and of the Council of 12 December 2006 on the protection of groundwater against pollution and deterioration, Official Journal of the European Union, L372, 19–31, 2006.
Fanning, A. L., O'Neill, D. W., Hickel, J., and Roux, N.: The social shortfall and ecological overshoot of nations, Nat. Sustain., 5, 26–36, https://doi.org/10.1038/s41893-021-00799-z, 2022.
FAO and ITPS: Status of the World's Soil Resources (SWSR) – Main Report, Food and Agriculture Organization of the United Nations and Intergovernmental Technical Panel on Soils, Rome, Italy, ISBN 978-92-5-109004-6, 2015.
Fierer, N., Wood, S. A., and Bueno de Mesquita, C. P.: How microbes can, and cannot, be used to assess soil health, Soil Biol. Biochem., 153, 108111, https://doi.org/10.1016/j.soilbio.2020.108111, 2021.
Froger, C., Jolivet, C., Budzinski, H., Pierdet, M., Caria, G., Saby, N. P. A., Arrouays, D., and Bispo, A.: Pesticide Residues in French Soils: Occurrence, Risks, and Persistence, Environ. Sci. Technol., 57, 7818–7827, https://doi.org/10.1021/acs.est.2c09591, 2023.
Fukami, T. and Nakajima, M.: Complex plant–soil interactions enhance plant species diversity by delaying community convergence, J. Ecol., 101, 316–324, https://doi.org/10.1111/1365-2745.12048, 2013.
Garland, G., Banerjee, S., Edlinger, A., Oliveira, E. M., Herzog, C., Wittwer, R., Philippot, L., Maestre, F. T., and Heijden, M. G. A. van der: A closer look at the functions behind ecosystem multifunctionality: A review, J. Ecol., 109, 600–613, https://doi.org/10.1111/1365-2745.13511, 2021.
Gatica, G., Fernández, M. E., Juliarena, M. P., and Gyenge, J.: Environmental and anthropogenic drivers of soil methane fluxes in forests: Global patterns and among-biomes differences, Glob. Change Biol., 26, 6604–6615, https://doi.org/10.1111/gcb.15331, 2020.
Gentile, R., Vanlauwe, B., Chivenge, P., and Six, J.: Interactive effects from combining fertilizer and organic residue inputs on nitrogen transformations, Soil Biol. Biochem., 40, 2375–2384, https://doi.org/10.1016/j.soilbio.2008.05.018, 2008.
Georgiou, K., Jackson, R. B., Vindušková, O., Abramoff, R. Z., Ahlström, A., Feng, W., Harden, J. W., Pellegrini, A. F. A., Polley, H. W., Soong, J. L., Riley, W. J., and Torn, M. S.: Global stocks and capacity of mineral-associated soil organic carbon, Nat. Commun., 13, 3797, https://doi.org/10.1038/s41467-022-31540-9, 2022.
Grace, J. B.: Structural equation modeling and natural systems, Cambridge University Press, https://doi.org/10.1017/CBO9780511617799, 2006.
Grace, J. B., Anderson, T. M., Olff, H., and Scheiner, S. M.: On the specification of structural equation models for ecological systems, Ecol. Monogr., 80, 67–87, https://doi.org/10.1890/09-0464.1, 2010.
Grace, J. B., Schoolmaster, D. R., Guntenspergen, G. R., Little, A. M., Mitchell, B. R., Miller, K. M., and Schweiger, E. W.: Guidelines for a graph-theoretic implementation of structural equation modeling, Ecosphere, 3, 73, https://doi.org/10.1890/ES12-00048.1, 2012.
Grime, P. J.: Plant strategies, vegetation processes, and ecosystem properties, 2nd Edn., John Wiley & Sons, Chichester, UK, ISBN 978-0-470-85040-4, 2001.
Hardin, G.: The competitive exclusion principle, Science, 131, 1292–1297, 1960.
Harris, J. A., Evans, D. L., and Mooney, S. J.: A new theory for soil health, Eur. J. Soil Sci., 73, e13292, https://doi.org/10.1111/ejss.13292, 2022.
Jöreskog, K. G.: Some contributions to maximum likelihood factor analysis, Psychometrika, 32, 443–482, 1967.
Keesstra, S., Geissen, V., Mosse, K., Piiranen, S., Scudiero, E., Leistra, M., and van Schaik, L.: Soil as a filter for groundwater quality, Curr. Opin. Environ. Sustain., 4, 507–516, https://doi.org/10.1016/j.cosust.2012.10.007, 2012.
Keesstra, S. D., Bouma, J., Wallinga, J., Tittonell, P., Smith, P., Cerdà, A., Montanarella, L., Quinton, J. N., Pachepsky, Y., van der Putten, W. H., Bardgett, R. D., Moolenaar, S., Mol, G., Jansen, B., and Fresco, L. O.: The significance of soils and soil science towards realization of the United Nations Sustainable Development Goals, SOIL, 2, 111–128, https://doi.org/10.5194/soil-2-111-2016, 2016.
Kou, X., Morriën, E., Tian, Y., Zhang, X., Lu, C., Xie, H., Liang, W., Li, Q., and Liang, C.: Exogenous carbon turnover within the soil food web strengthens soil carbon sequestration through microbial necromass accumulation, Glob. Change Biol., 29, 4069–4080, https://doi.org/10.1111/gcb.16749, 2023.
Lade, S. J., Steffen, W., de Vries, W., Carpenter, S. R., Donges, J. F., Gerten, D., Hoff, H., Newbold, T., Richardson, K., and Rockström, J.: Human impacts on planetary boundaries amplified by Earth system interactions, Nat. Sustain., 3, 119–128, https://doi.org/10.1038/s41893-019-0454-4, 2020.
Lal, R.: Soil organic matter and water retention, Agron. J., 112, 3265–3277, https://doi.org/10.1002/agj2.20282, 2020.
Lal, R., Bouma, J., Brevik, E., Dawson, L., Field, D. J., Glaser, B., Hatano, R., Hartemink, A. E., Kosaki, T., Lascelles, B., Monger, C., Muggler, C., Ndzana, G. M., Norra, S., Pan, X., Paradelo, R., Reyes-Sánchez, L. B., Sandén, T., Singh, B. R., Spiegel, H., Yanai, J., and Zhang, J.: Soils and sustainable development goals of the United Nations: An International Union of Soil Sciences perspective, Geoderma Reg., 25, e00398, https://doi.org/10.1016/j.geodrs.2021.e00398, 2021.
Lamontagne, X. and Shipley, B.: A measure of generalized soil fertility that is largely independent of species identity, Ann. Bot., 129, 29–36, https://doi.org/10.1093/aob/mcab121, 2022.
Laub, M., Schlichenmeier, S., Vityakon, P., and Cadisch, G.: Litter Quality and Microbes Explain Aggregation Differences in a Tropical Sandy Soil, J. Soil Sci. Plant Nutr., 22, 848–860, https://doi.org/10.1007/s42729-021-00696-6, 2022.
Lavallee, J. M., Soong, J. L., and Cotrufo, M. F.: Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century, Glob. Change Biol., 26, 261–273, https://doi.org/10.1111/gcb.14859, 2020.
Leclère, D., Obersteiner, M., Barrett, M., Butchart, S. H. M., Chaudhary, A., De Palma, A., DeClerck, F. A. J., Di Marco, M., Doelman, J. C., Dürauer, M., Freeman, R., Harfoot, M., Hasegawa, T., Hellweg, S., Hilbers, J. P., Hill, S. L. L., Humpenöder, F., Jennings, N., Krisztin, T., Mace, G. M., Ohashi, H., Popp, A., Purvis, A., Schipper, A. M., Tabeau, A., Valin, H., van Meijl, H., van Zeist, W.-J., Visconti, P., Alkemade, R., Almond, R., Bunting, G., Burgess, N. D., Cornell, S. E., Di Fulvio, F., Ferrier, S., Fritz, S., Fujimori, S., Grooten, M., Harwood, T., Havlík, P., Herrero, M., Hoskins, A. J., Jung, M., Kram, T., Lotze-Campen, H., Matsui, T., Meyer, C., Nel, D., Newbold, T., Schmidt-Traub, G., Stehfest, E., Strassburg, B. B. N., van Vuuren, D. P., Ware, C., Watson, J. E. M., Wu, W., and Young, L.: Bending the curve of terrestrial biodiversity needs an integrated strategy, Nature, 585, 551–556, https://doi.org/10.1038/s41586-020-2705-y, 2020.
Lehmann, A. and Stahr, K.: The potential of soil functions and planner-oriented soil evaluation to achieve sustainable land use, J. Soils Sediments, 10, 1092–1102, https://doi.org/10.1007/s11368-010-0207-5, 2010.
Lehmann, J. and Kleber, M.: The contentious nature of soil organic matter, Nature, 528, 60–68, https://doi.org/10.1038/nature16069, 2015.
Lehmann, J., Bossio, D. A., Kögel-Knabner, I., and Rillig, M. C.: The concept and future prospects of soil health, Nat. Rev. Earth Environ., 1–10, https://doi.org/10.1038/s43017-020-0080-8, 2020.
Levy, P. E., Burden, A., Cooper, M. D. A., Dinsmore, K. J., Drewer, J., Evans, C., Fowler, D., Gaiawyn, J., Gray, A., Jones, S. K., Jones, T., McNamara, N. P., Mills, R., Ostle, N., Sheppard, L. J., Skiba, U., Sowerby, A., Ward, S. E., and Zieliński, P.: Methane emissions from soils: synthesis and analysis of a large UK data set, Glob. Change Biol., 18, 1657–1669, https://doi.org/10.1111/j.1365-2486.2011.02616.x, 2012.
Maaz, T. M., Heck, R. H., Glazer, C. T., Loo, M. K., Zayas, J. R., Krenz, A., Beckstrom, T., Crow, S. E., and Deenik, J. L.: Measuring the immeasurable: A structural equation modeling approach to assessing soil health, Sci. Total Environ., 870, 161900, https://doi.org/10.1016/j.scitotenv.2023.161900, 2023.
Mack, K. M. L. and Bever, J. D.: Coexistence and relative abundance in plant communities are determined by feedbacks when the scale of feedback and dispersal is local, J. Ecol., 102, 1195–1201, https://doi.org/10.1111/1365-2745.12269, 2014.
Mack, K. M. L., Eppinga, M. B., and Bever, J. D.: Plant-soil feedbacks promote coexistence and resilience in multi-species communities, PLOS ONE, 14, e0211572, https://doi.org/10.1371/journal.pone.0211572, 2019.
Manning, P., van der Plas, F., Soliveres, S., Allan, E., Maestre, F. T., Mace, G., Whittingham, M. J., and Fischer, M.: Redefining ecosystem multifunctionality, Nat. Ecol. Evol., 2, 427, https://doi.org/10.1017/CBO9780511803161, 2018.
Montanarella, L. and Panagos, P.: The relevance of sustainable soil management within the European Green Deal, Land Use Policy, 100, 104950, https://doi.org/10.1016/j.landusepol.2020.104950, 2021.
Nagajyoti, P. C., Lee, K. D., and Sreekanth, T. V. M.: Heavy metals, occurrence and toxicity for plants: a review, Environ. Chem. Lett., 8, 199–216, https://doi.org/10.1007/s10311-010-0297-8, 2010.
Nolan, B. T. and Stoner, J. D.: Nutrients in Groundwaters of the Conterminous United States, 1992–1995, Environ. Sci. Technol., 34, 1156–1165, https://doi.org/10.1021/es9907663, 2000.
Norris, C. E., Bean, G. M., Cappellazzi, S. B., Cope, M., Greub, K. L. H., Liptzin, D., Rieke, E. L., Tracy, P. W., Morgan, C. L. S., and Honeycutt, C. W.: Introducing the North American project to evaluate soil health measurements, Agron. J., 112, 3195–3215, https://doi.org/10.1002/agj2.20234, 2020.
Orgiazzi, A., Panagos, P., Fernández-Ugalde, O., Wojda, P., Labouyrie, M., Ballabio, C., Franco, A., Pistocchi, A., Montanarella, L., and Jones, A.: LUCAS Soil Biodiversity and LUCAS Soil Pesticides, new tools for research and policy development, Eur. J. Soil Sci., 73, e13299, https://doi.org/10.1111/ejss.13299, 2022.
Pearl, J.: Causality, Cambridge University Press, 487 pp., https://doi.org/10.1017/CBO9780511803161, 2009.
Pearson, K.: The grammar of science, 3rd Edn., Adam & Charles Black, London, 567 pp., 1911.
Pereira, H. M., Leadley, P. W., Proença, V., Alkemade, R., Scharlemann, J. P. W., Fernandez-Manjarrés, J. F., Araújo, M. B., Balvanera, P., Biggs, R., Cheung, W. W. L., Chini, L., Cooper, H. D., Gilman, E. L., Guénette, S., Hurtt, G. C., Huntington, H. P., Mace, G. M., Oberdorff, T., Revenga, C., Rodrigues, P., Scholes, R. J., Sumaila, U. R., and Walpole, M.: Scenarios for Global Biodiversity in the 21st Century, Science, 330, 1496–1501, https://doi.org/10.1126/science.1196624, 2010.
Pereira, J. L. J., Oliver, G. A., Francisco, M. B., Cunha, S. S., and Gomes, G. F.: A Review of Multi-objective Optimization: Methods and Algorithms in Mechanical Engineering Problems, Arch. Comput. Methods Eng., 29, 2285–2308, https://doi.org/10.1007/s11831-021-09663-x, 2022.
Petchey, O. L., Pontarp, M., Massie, T. M., Kéfi, S., Ozgul, A., Weilenmann, M., Palamara, G. M., Altermatt, F., Matthews, B., Levine, J. M., Childs, D. Z., McGill, B. J., Schaepman, M. E., Schmid, B., Spaak, P., Beckerman, A. P., Pennekamp, F., and Pearse, I. S.: The ecological forecast horizon, and examples of its uses and determinants, Ecol. Lett., 18, 597–611, https://doi.org/10.1111/ele.12443, 2015.
Pielou, E. C.: The measurement of diversity in different types of biological collections, J. Theor. Biol., 13, 131–144, https://doi.org/10.1016/0022-5193(66)90013-0, 1966.
Rosseel, Y.: lavaan: An R Package for Structural Equation Modeling, J. Stat. Softw., 48, 1–36, https://doi.org/10.18637/jss.v048.i02, 2012.
Sagova-Mareckova, M., Omelka, M., and Kopecky, J.: The Golden Goal of Soil Management: Disease-Suppressive Soils, Phytopathology®, PHYTO-09-22-0324-KD, https://doi.org/10.1094/PHYTO-09-22-0324-KD, 2022.
Schadt, C. W., Martin, A. P., Lipson, D. A., and Schmidt, S. K.: Seasonal dynamics of previously unknown fungal lineages in tundra soils, Science, 301, 1359–1361, https://doi.org/10.1126/science.1086940, 2003.
Schreefel, L., de Boer, I. J. M., Timler, C. J., Groot, J. C. J., Zwetsloot, M. J., Creamer, R. E., Schrijver, A. P., van Zanten, H. H. E., and Schulte, R. P. O.: How to make regenerative practices work on the farm: A modelling framework, Agric. Syst., 198, 103371, https://doi.org/10.1016/j.agsy.2022.103371, 2022.
Schröder, J. J., Schulte, R. P. O., Creamer, R. E., Delgado, A., Leeuwen, J. van, Lehtinen, T., Rutgers, M., Spiegel, H., Staes, J., Tóth, G., and Wall, D. P.: The elusive role of soil quality in nutrient cycling: a review, Soil Use Manag., 32, 476–486, https://doi.org/10.1111/sum.12288, 2016.
Schulte, R. P. O., Creamer, R. E., Donnellan, T., Farrelly, N., Fealy, R., O'Donoghue, C., and O'hUallachain, D.: Functional land management: A framework for managing soil-based ecosystem services for the sustainable intensification of agriculture, Environ. Sci. Policy, 38, 45–58, https://doi.org/10.1016/j.envsci.2013.10.002, 2014.
Schulte, R. P. O., O'Sullivan, L., Vrebos, D., Bampa, F., Jones, A., and Staes, J.: Demands on land: Mapping competing societal expectations for the functionality of agricultural soils in Europe, Environ. Sci. Policy, 100, 113–125, https://doi.org/10.1016/j.envsci.2019.06.011, 2019.
Shipley, B.: A New Inferential Test for Path Models Based on Directed Acyclic Graphs, Struct. Equ. Model. Multidiscip. J., 7, 206–218, https://doi.org/10.1207/S15328007SEM0702_4, 2000.
Shipley, B.: Cause and correlation in biology: a user's guide to path analysis, structural equations, and causal inference with R, Cambridge University Press, Cambridge, UK, 229 pp., https://doi.org/10.1017/CBO9781139979573, 2016.
Sigl, L., Falkenberg, R., and Fochler, M.: Changing articulations of relevance in soil science: Diversity and (potential) synergy of epistemic commitments in a scientific discipline, Stud. Hist. Philos. Sci., 97, 79–90, https://doi.org/10.1016/j.shpsa.2022.12.004, 2023.
Siwicka, E., Gladstone-Gallagher, R., Hewitt, J. E., and Thrush, S. F.: Beyond the single index: Investigating ecological mechanisms underpinning ecosystem multifunctionality with network analysis, Ecol. Evol., 11, 12401–12412, https://doi.org/10.1002/ece3.7987, 2021.
Six, J., Elliott, E. T., and Paustian, K.: Soil macroaggregate turnover and microaggregate formation: a mechanism for C sequestration under no-tillage agriculture, Soil Biol. Biochem., 32, 2099–2103, https://doi.org/10.1016/S0038-0717(00)00179-6, 2000.
Sokol, N. W., Slessarev, E., Marschmann, G. L., Nicolas, A., Blazewicz, S. J., Brodie, E. L., Firestone, M. K., Foley, M. M., Hestrin, R., Hungate, B. A., Koch, B. J., Stone, B. W., Sullivan, M. B., Zablocki, O., and Pett-Ridge, J.: Life and death in the soil microbiome: how ecological processes influence biogeochemistry, Nat. Rev. Microbiol., 1–16, https://doi.org/10.1038/s41579-022-00695-z, 2022.
Spearman, C.: General Intelligence objectively determined and measured., Am. J. Psychol., 15, 201–93, 1904.
Springmann, M., Clark, M., Mason-D'Croz, D., Wiebe, K., Bodirsky, B. L., Lassaletta, L., de Vries, W., Vermeulen, S. J., Herrero, M., Carlson, K. M., Jonell, M., Troell, M., DeClerck, F., Gordon, L. J., Zurayk, R., Scarborough, P., Rayner, M., Loken, B., Fanzo, J., Godfray, H. C. J., Tilman, D., Rockström, J., and Willett, W.: Options for keeping the food system within environmental limits, Nature, 562, 519–525, https://doi.org/10.1038/s41586-018-0594-0, 2018.
Steffen, W., Richardson, K., Rockström, J., Cornell, S. E., Fetzer, I., Bennett, E. M., Biggs, R., Carpenter, S. R., Vries, W. de, Wit, C. A. de, Folke, C., Gerten, D., Heinke, J., Mace, G. M., Persson, L. M., Ramanathan, V., Reyers, B., and Sörlin, S.: Planetary boundaries: Guiding human development on a changing planet, Science, 347, 1259855, https://doi.org/10.1126/science.1259855, 2015.
Stoof, C. R., Moore, D., Ritsema, C. J., and Dekker, L. W.: Natural and fire-induced soil water repellency in a Portuguese shrubland, Soil Sci. Soc. Am. J., 75, 2283–2295, https://doi.org/10.2136/sssaj2011.0046, 2011.
Sun, X., Liddicoat, C., Tiunov, A., Wang, B., Zhang, Y., Lu, C., Li, Z., Scheu, S., Breed, M. F., Geisen, S., and Zhu, Y.-G.: Harnessing soil biodiversity to promote human health in cities, Npj Urban Sustain., 3, 1–8, https://doi.org/10.1038/s42949-023-00086-0, 2023.
Tang, F. H. M. and Maggi, F.: Pesticide mixtures in soil: a global outlook, Environ. Res. Lett., 16, 044051, https://doi.org/10.1088/1748-9326/abe5d6, 2021.
Thiele-Bruhn, S.: The role of soils in provision of genetic, medicinal and biochemical resources, Philos. Trans. R. Soc. B Biol. Sci., 376, 20200183, https://doi.org/10.1098/rstb.2020.0183, 2021.
Tian, H., Xu, R., Canadell, J. G., Thompson, R. L., Winiwarter, W., Suntharalingam, P., Davidson, E. A., Ciais, P., Jackson, R. B., Janssens-Maenhout, G., Prather, M. J., Regnier, P., Pan, N., Pan, S., Peters, G. P., Shi, H., Tubiello, F. N., Zaehle, S., Zhou, F., Arneth, A., Battaglia, G., Berthet, S., Bopp, L., Bouwman, A. F., Buitenhuis, E. T., Chang, J., Chipperfield, M. P., Dangal, S. R. S., Dlugokencky, E., Elkins, J. W., Eyre, B. D., Fu, B., Hall, B., Ito, A., Joos, F., Krummel, P. B., Landolfi, A., Laruelle, G. G., Lauerwald, R., Li, W., Lienert, S., Maavara, T., MacLeod, M., Millet, D. B., Olin, S., Patra, P. K., Prinn, R. G., Raymond, P. A., Ruiz, D. J., van der Werf, G. R., Vuichard, N., Wang, J., Weiss, R. F., Wells, K. C., Wilson, C., Yang, J., and Yao, Y.: A comprehensive quantification of global nitrous oxide sources and sinks, Nature, 586, 248–256, https://doi.org/10.1038/s41586-020-2780-0, 2020.
Trajanov, A., Spiegel, H., Debeljak, M., and Sandén, T.: Using data mining techniques to model primary productivity from international long-term ecological research (ILTER) agricultural experiments in Austria, Reg. Environ. Change, 19, 325–337, https://doi.org/10.1007/s10113-018-1361-3, 2019.
Van de Broek, M., Henriksen, C. B., Ghaley, B. B., Lugato, E., Kuzmanovski, V., Trajanov, A., Debeljak, M., Sandén, T., Spiegel, H., Decock, C., Creamer, R., and Six, J.: Assessing the Climate Regulation Potential of Agricultural Soils Using a Decision Support Tool Adapted to Stakeholders' Needs and Possibilities, Front. Environ. Sci., 7, art131, https://doi.org/10.3389/fenvs.2019.00131, 2019.
Van der Putten, W. H., Bardgett, R. D., Bever, J. D., Bezemer, T. M., Casper, B. B., Fukami, T., Kardol, P., Klironomos, J. N., Kulmatiski, A., Schweitzer, J. A., Suding, K. N., Van de Voorde, T. F. J., and Wardle, D. A.: Plant–soil feedbacks: the past, the present and future challenges, J. Ecol., 101, 265–276, https://doi.org/10.1111/1365-2745.12054, 2013.
Van der Putten, W. H., Bardgett, R. D., Farfan, M., Montanarella, L., Six, J., and Wall, D. H.: Soil biodiversity needs policy without borders, Science, 379, 32–34, https://doi.org/10.1126/science.abn7248, 2023.
Van Leeuwen, J. P., Saby, N. P. A., Jones, A., Louwagie, G., Micheli, E., Rutgers, M., Schulte, R. P. O., Spiegel, H., Toth, G., and Creamer, R. E.: Gap assessment in current soil monitoring networks across Europe for measuring soil functions, Environ. Res. Lett., 12, 124007, https://doi.org/10.1088/1748-9326/aa9c5c, 2017.
Vance, E. D., Brookes, P. C., and Jenkinson, D. S.: An extraction method for measuring soil microbial biomass C, Soil Biol. Biochem., 19, 703–707, https://doi.org/10.1016/0038-0717(87)90052-6, 1987.
Veerman, C., Pinto Correia, T., Bastioli, C., Biro, B., Bouma, J., Emmett, B., Frison, E. A., Grand, A., Hristov Filchew, L., Kriaučiūnienė, Z., Pogrzeba, M., Soussana, J.-F., Vela Olmo, C., and Wittkowski, R.: Caring for soil is caring for life – Ensure 75 % of soils are healthy by 2030 for food, people, nature and climate, European Commission, https://doi.org/10.2777/611303, Brussels, 2020.
Vogel, H.-J., Bartke, S., Daedlow, K., Helming, K., Kögel-Knabner, I., Lang, B., Rabot, E., Russell, D., Stößel, B., Weller, U., Wiesmeier, M., and Wollschläger, U.: A systemic approach for modeling soil functions, SOIL, 4, 83–92, https://doi.org/10.5194/soil-4-83-2018, 2018.
de Vries, W., Lofts, S., Tipping, E., Meili, M., Groenenberg, J. E., and Schütze, G.: Impact of Soil Properties on Critical Concentrations of Cadmium, Lead, Copper, Zinc, and Mercury in Soil and Soil Solution in View of Ecotoxicological Effects, in: Reviews of Environmental Contamination and Toxicology, Springer, New York, NY, 47–89, https://doi.org/10.1007/978-0-387-69163-3_3, 2007.
Wagg, C., Bender, S. F., Widmer, F., and Heijden, M. G. A. van der: Soil biodiversity and soil community composition determine ecosystem multifunctionality, P. Natl. Acad. Sci. USA, 111, 5266–5270, https://doi.org/10.1073/pnas.1320054111, 2014.
Wall, D. H., Nielsen, U. N., and Six, J.: Soil biodiversity and human health, Nature, 528, 69–76, https://doi.org/10.1038/nature15744, 2015.
Wall, D. P., Delgado, A., O'Sullivan, L., Creamer, R. E., Trajanov, A., Kuzmanovski, V., Bugge Henriksen, C., and Debeljak, M.: A Decision Support Model for Assessing the Water Regulation and Purification Potential of Agricultural Soils Across Europe, Front. Sustain. Food Syst., 4, 115, https://doi.org/10.3389/fsufs.2020.00115, 2020.
Wiesmeier, M., Urbanski, L., Hobley, E., Lang, B., von Lützow, M., Marin-Spiotta, E., van Wesemael, B., Rabot, E., Ließ, M., Garcia-Franco, N., Wollschläger, U., Vogel, H.-J., and Kögel-Knabner, I.: Soil organic carbon storage as a key function of soils – A review of drivers and indicators at various scales, Geoderma, 333, 149–162, https://doi.org/10.1016/j.geoderma.2018.07.026, 2019.
Van Wijnen, H. J., Rutgers, M., Schouten, A. J., Mulder, C., de Zwart, D., and Breure, A. M.: How to calculate the spatial distribution of ecosystem services – Natural attenuation as example from The Netherlands, Sci. Total Environ., 415, 49–55, https://doi.org/10.1016/j.scitotenv.2011.05.058, 2012.
Wright, S.: Correlation and causation, J. Agric. Res., 20, 557–585, 1921.
Wright, S.: The Method of Path Coefficients, Ann. Math. Stat., 5, 161–215, 1934.
Wubs, E. R. J.: SoilMFv0.1, [code], Zenodo, https://doi.org/10.5281/zenodo.16946924, 2025.
Wubs, E. R. J. and Nugteren, J.: Data for the generalized soil fertility test in Dutch soils, figshare [data set], https://doi.org/10.6084/m9.figshare.29132744.v1, 2025.
Young, I. M. and Crawford, J. W.: Interactions and Self-Organization in the Soil-Microbe Complex, Science, 304, 1634–1637, https://doi.org/10.1126/science.1097394, 2004.
Zhou, M., Zhu, B., Wang, S., Zhu, X., Vereecken, H., and Brüggemann, N.: Stimulation of N2O emission by manure application to agricultural soils may largely offset carbon benefits: a global meta-analysis, Glob. Change Biol., 23, 4068–4083, https://doi.org/10.1111/gcb.13648, 2017.
Zwetsloot, M. J., Leeuwen, J. van, Hemerik, L., Martens, H., Josa, I. S., Broek, M. V. de, Debeljak, M., Rutgers, M., Sandén, T., Wall, D. P., Jones, A., and Creamer, R. E.: Soil multifunctionality: Synergies and trade-offs across European climatic zones and land uses, Eur. J. Soil Sci., 72, 1640–1654, https://doi.org/10.1111/ejss.13051, 2021.
- Abstract
- Introduction
- Conceptual approach to soil multifunctionality
- Box 1. Causal inference, structural equation modelling, and latent variables – a short introduction
- Selecting soil functions and boundary conditions
- The IQ test for soils – a proposal
- Practical implementation in science and beyond
- Discussion
- Conclusions
- Code availability
- Data availability
- Author contributions
- Competing interests
- Disclaimer
- Acknowledgements
- Financial support
- Review statement
- References
- Supplement
- Abstract
- Introduction
- Conceptual approach to soil multifunctionality
- Box 1. Causal inference, structural equation modelling, and latent variables – a short introduction
- Selecting soil functions and boundary conditions
- The IQ test for soils – a proposal
- Practical implementation in science and beyond
- Discussion
- Conclusions
- Code availability
- Data availability
- Author contributions
- Competing interests
- Disclaimer
- Acknowledgements
- Financial support
- Review statement
- References
- Supplement