the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Contrasting potential for biological N2 fixation at three polluted central European Sphagnum peat bogs: combining the 15N2-tracer and natural-abundance isotope approaches
Marketa Stepanova
Bohuslava Cejkova
Ivana Jackova
Frantisek Buzek
Frantisek Veselovsky
Jan Curik
Eva Prechova
Arnost Komarek
Leona Bohdalkova
Availability of reactive nitrogen (Nr) is a key control on carbon (C) sequestration in wetlands. To complement the metabolic demands of Sphagnum in pristine rain-fed bogs, diazotrophs supply additional Nr via biological nitrogen fixation (BNF). As breaking the triple bond of atmospheric N2 is energy-intensive, it is reasonable to assume that increasing inputs of pollutant Nr will lead to BNF downregulation. However, recent studies have also documented measurable BNF rates in Sphagnum-dominated bogs in polluted regions, indicating the adaptation of N2 fixers to changing N deposition. Our aim was to quantify BNF in high-elevation peatlands located in industrialized central Europe. A 15N2-tracer experiment was combined with a natural-abundance N-isotope study at three Sphagnum-dominated peat bogs in the northern Czech Republic in an attempt to assess the roles of individual BNF drivers. High short-term BNF rates (8.2 ± 4.6 g N m2 d−1) were observed at Malé mechové jezírko, which receives ∼ 17 kg Nr ha−1 yr−1. The remaining two peat bogs, whose recent atmospheric Nr inputs differed from Malé mechové jezírko by only 1–2 kg ha−1 yr−1 (Uhlír̆ská and Brumiště), showed zero BNF. The following parameters were investigated to elucidate the BNF difference: the NH-N NO-N ratio, temperature, wetness, Sphagnum species, organic-N availability, possible P limitation, possible molybdenum (Mo) limitation, SO deposition, and pH. At Malé mechové jezírko and Uhlír̆ská, the same moss species (S. girgensohnii) was used for the 15N2 experiment; therefore, the host identity could not explain the difference in BNF at these sites. Temperature and moisture were also identical in all incubations and could not explain the between-site differences in BNF. The N : P stoichiometry in peat and bog water indicated that Brumiště may have lacked BNF due to P limitation, whereas non-detectable BNF at Uhlír̆ská may have been related to the 70-fold higher SO concentration in bog water. Across the sites, the mean natural-abundance δ15N values increased in the following order: atmospheric deposition (−5.3 ± 0.3 ‰) < Sphagnum (−4.3 ± 0.1 ‰) < bog water (−3.9 ± 0.4 ‰) < atmospheric N2 (0.0 ‰). Only at Brumiště was N in Sphagnum significantly isotopically heavier than in atmospheric deposition, possibly indicating a longer-term BNF effect. Collectively, our data highlight spatial heterogeneity in BNF rates under high Nr inputs as well as the importance of environmental parameters other than atmospheric Nr pollution in regulating BNF.
- Article
(2937 KB) - Full-text XML
-
Supplement
(2519 KB) - BibTeX
- EndNote
Nitrogen (N) is the limiting nutrient in most terrestrial environments. The amount and form of N available to organisms (reactive N, Nr) is controlled by biogeochemical processes (Vitousek and Howarth, 1991; LeBauer and Treseder, 2008; Zhang et al., 2020; Davies-Barnard and Friedlingstein, 2020). A growing body of research has focused on the role of biological N2 fixation (BNF) as a source of Nr in pristine ecosystems, such as subarctic tundra and boreal forests, with special attention given to ombrotrophic peat bogs and minerotrophic fens (Hemond, 1983; Rousk et al., 2013, 2015; Larmola et al., 2014; Vile et al., 2014; Diakova et al., 2016; Stuart et al., 2021; Yin et al., 2022). Globally, peatlands store between 20 % and 30 % of total soil carbon and approximately 15 % of total soil nitrogen (Wieder and Vitt, 2006; Gallego-Sala et al., 2018; Fritz et al., 2014). Microbial N2 fixation helps to sustain C accumulation in peatlands and to remove carbon dioxide (CO2) from the atmosphere (Vile et al., 2014, and references therein). Changes in BNF may affect the dynamics of climate change. A combination of high anthropogenic Nr inputs and sustained N2 fixation may accelerate the invasion of vascular plants into peat bogs, leading to a reduction of C–N stocks.
The nitrogen budget at the peat bog scale results from a balance between N inputs – atmospheric deposition of Nr, mostly nitrate (NO) and ammonium (NH), with a contribution of organic N and BNF – and N outputs – runoff dominated by dissolved, colloidal, and particulate N, and emissions of gaseous N forms, mainly nitrous oxide (N2O), nitric oxide (NO), and N2 as products of denitrification (Sgouridis et al., 2021). The atmospheric lifetime of N2O, a potent greenhouse gas, is relatively long (> 100 years; Frolking et al., 2011). In contrast, the atmospheric lifetime of NO, another greenhouse gas, is short (days), and, along with N2 as the final product of denitrification with no warming potential, is not considered in climate warming scenarios. Atmospheric deposition of Nr in high-latitude pristine bogs is 0.5–1.0 kg ha−1yr−1 (Vitt et al., 2003). Bogs receiving less than 10 kg Nr ha−1 yr−1 are defined as having low levels of pollution (Lamers et al., 2000). Bogs receiving more than 18 kg Nr ha−1 yr−1 are considered to be highly polluted. Reactive N deposited on the surface of ombrotrophic peat bogs is vertically mobile (Novak et al., 2014).
Nitrogen capture in rain-fed bogs is dominated by Sphagnum mosses (Limpens et al., 2006). Nitrogen-fixing microbes (diazotrophs) mostly reside inside specialized Sphagnum cells (hyalocytes), although the mosses' metabolic demands for N are also supported by free-living diazotrophs. In contrast, diazotrophs in feather mosses, common in boreal forests, live epiphytically on the leaves (DeLuca et al., 2002; Rousk et al., 2015). Endophytic diazotrophs are more protected against environmental fluctuations, including changes in Nr deposition. BNF in bogs is associated mostly with cyanobacteria and methanotrophs (Larmola et al., 2014; Vile et al., 2014; Leppanen et al., 2015; Holland-Moritz et al., 2021; Kolton et al., 2022). It follows that BNF may affect potential methane (CH4) emissions in two opposing directions: while higher C accumulation due to efficient BNF may lead to higher CH4 emissions during peat decomposition, N2-fixing methanotrophs may reduce emissions of CH4 by oxidizing this greenhouse gas.
Recent work in peatlands has quantified the relative roles of various biotic and abiotic controls over BNF. Leppanen et al. (2015) reported than BNF rates were independent of the diazotroph community structure. The effect of temperature was reviewed by Carrell et al. (2019), Zivkovic et al. (2022), and Yin et al. (2022). The optimal temperature for BNF is 20–30 ∘C (Zielke et al., 2005). Dry conditions are generally unfavorable for BNF, but the moisture–BNF correlation tends to be insignificant (Yin et al., 2022). The effect of phosphorus (P) as a limiting nutrient was evaluated by Limpens et al. (2004), Larmola et al. (2014), Ho and Bodelier (2015), van den Elzen et al. (2017, 2020), and Zivkovic et al. (2022). In an interplay with other environmental and chemical parameters, higher P availability may augment BNF. The role of the NH NO ratio in atmospheric deposition as a BNF control was evaluated by Saiz et al. (2021). A higher NH proportion relative to the total Nr deposition may result in lower BNF rates. Stuart et al. (2021) stressed a strong interaction between moss identity, temperature, moisture, and pH as possible BNF drivers. Kox et al. (2018) reported higher BNF rates as a result of oxygen (O2) depletion. Wieder et al. (2019, 2020) and Kox et al. (2020) showed that BNF rates generally increase in the presence of light.
In previous studies, BNF rates were measured under field conditions (e.g., Vile et al., 2014; Rousk et al., 2017; van den Elzen et al., 2020; Saiz et al., 2021; Zivkovic et al., 2022) or under controlled laboratory conditions (e.g., Knorr et al., 2015; van den Elzen et al., 2017; Warren et al., 2017; Stuart et al., 2021). According to Myrold et al. (1999), an advantage of laboratory 15N2 experiments is related to easier preservation of a gastight assay system. The rates of BNF are measured using an acetylene reduction assay (ARA), 15N2 isotope-labeling incubations, or compound-specific amino acid 15N probing (e.g., Knorr et al., 2015; Chiewattanakul et al., 2022). Recent studies have stressed the need for caution in ARA studies (Vile et al., 2014; Saiz et al., 2019; Soper et al., 2021). Inhibition of the activity of methanotrophs by acetylene may lead to an underestimation of BNF rates. These methods of direct measurements inevitably choose specific experimental conditions and, thus, provide potential instantaneous BNF rates. A complementary, indirect evaluation of BNF can be based on natural-abundance 15N 14N isotope systematics (Novak et al., 2016; Zivkovic et al., 2017; Saiz et al., 2021; Stuart et al., 2021). Sphagnum taking up N through BNF would carry a δ15N signature close to 0 ‰, a value characterizing atmospheric N2 (δ15N values are defined as a per mil deviation of the 15N 14N ratio in the sample from a standard; the widely used standard is atmospheric N2). With increasing BNF rates, the δ15N values of living Sphagnum converge from the often negative δ15N value of atmospheric deposition to the 0 ‰ value of the source N2. This simple approach is complicated by tight inner N cycling near the bog surface, involving open-system isotope fractionations. In particular, Sphagnum may additionally take up Nr resulting from the mineralization of organic N. Because denitrification preferentially removes isotopically light N in a gaseous form, the residual Nr in bog water may become isotopically heavy and supply high-δ15N nitrogen for assimilation. Thus, mineralized Nr in bog water as another nutrient source may be isotopically similar to atmospheric N2 (Novak et al., 2019; Stuart et al., 2021).
BNF is an energy-intensive process requiring 16 adenosine triphosphate (ATP) molecules to fix 1 mol of N2. It follows that, with an increasing input of pollutant Nr via atmospheric deposition, BNF should be rapidly downregulated. However, experiments applying additional Nr to Sphagnum (both in the laboratory and in the field) have indicated contradictory impacts on BNF. Some studies have shown a decrease in BNF rates in the proximity of anthropogenic Nr sources (Wieder et al., 2019; Saiz et al., 2021), whereas others have indicated continuing BNF even at N-polluted sites (van den Elzen et al., 2018). BNF data from natural settings with known time series of historical Nr deposition rates are rare (van den Elzen et al., 2018; Saiz et al., 2021). The aim of the current study was to quantify BNF at high-elevation Sphagnum-dominated peatlands in an industrial part of central Europe that is also known for intense agriculture. We combined 15N2-tracer experiments with a natural-abundance N-isotope study at three peat bogs situated in the northern Czech Republic to provide qualitative insights into the roles of individual BNF drivers. Our specific objectives were as follows: (i) to investigate whether BNF rates at the study sites correlate with well-constrained NO and NH deposition rates and P availability and (ii) to compare the results of experiments investigating 15N assimilation by Sphagnum with the results of a natural-abundance δ15N inventory of individual wetland pools and fluxes. We expected that convergence of Sphagnum N toward δ15N ‰ would corroborate the relative magnitude of instantaneous BNF rates in between-site comparisons. Because, thus far, the natural-abundance 15N approach has been rarely adopted in BNF studies, compared with the more frequently used 15N2-labeling approach, we generated a larger δ15N data set in the natural-abundance 15N monitoring part of our study.
2.1 Study sites
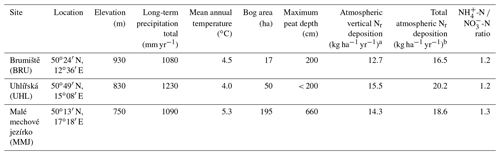
The three studied Sphagnum-dominated peat bogs (Fig. 1, Table 1) are located in the northern Czech Republic, a highly industrialized part of central Europe with numerous coal-burning power plants. In the period from the 1970s to the 1990s, Norway spruce monocultures were affected by acid rain in the vicinity of Brumiště (BRU; Ore Mountains) and Uhlír̆ská (UHL; Jizerské Mountains). At UHL, most spruce stands died back and were harvested. The third site, Malé mechové jezírko (MMJ; Jeseníky Mountains), is surrounded by relatively healthy mature spruce forests. The distance between adjacent study sites is 160–190 km (Fig. 1). The studied high-elevation catchments are drained by small streams. The peatlands are partly rain-fed, with a possible contribution of lateral water influx from the surrounding segments of the catchments. The bedrock is composed of granite at BRU and UHL and by phyllite at MMJ. The surface of each bog is characterized by a combination of hummock–hollow microtopography and lawns (Dohnal, 1965). Moss species at BRU include S. cuspidatum, common in hollows and pools; S. magellanicum, mostly occupying intermediate positions between the tops of the hummocks and the hollows; S. rubellum, typical of dense carpets in rain-fed bogs; and S. papillosum, forming low hummocks and mats in bogs and mires. At UHL and MMJ, the predominant moss species is shade-demanding S. girgensohnii, requiring slight base enrichment (Table S1 in the Supplement). The growing season is more than 7 months long, from late March to early November. The density of living Sphagnum is 0.04 g cm−3. More details on BRU are given in Bohdalkova et al. (2013) and Buzek et al. (2019, 2020). Biogeochemical processes at UHL have been studied by Novak et al. (2005), Sanda and Cislerova (2009), Bohdalkova et al. (2014), Marx et al. (2017), Oulehle et al. (2017, 2021a), and Vitvar et al. (2022). Further information on MMJ is given in Novak et al. (2003, 2009).
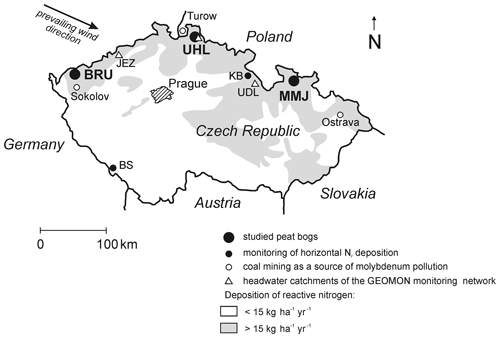
Figure 1Location of the studied Sphagnum-dominated peat bogs. Nr deposition contours are by Czech Hydrometeorological Institute (1998).
2.2 Sampling
In our study, we compared long-term N-isotope data (natural-abundance 15N monitoring in the field) with short-term N-isotope data (15N2 laboratory moss incubations).
Samples of rain and snow for δ15N determinations were collected between January 2016 and October 2019 using a simplified protocol based on that of Fottova and Skorepova (1998). Open-area precipitation was sampled by two rain collectors placed 5 m apart, 160 cm above ground. Spruce canopy throughfall was sampled using five (UHL) or three (BRU and MMJ) collectors installed 10 m apart. Deposition samplers were polyethylene (PE) funnels (surface area of 113 cm2) fitted to 1 L bottles. In winter, cylindrical PE vessels (surface area of 167 cm2) were used to collect snow. At the end of the cumulative 1-month sampling campaign, respective open-area precipitation and throughfall samples were pooled prior to chemical and N-isotope analysis. One-liter samples of runoff were collected in ∼ 30 d intervals at BRU over a 25-month period; samples of runoff were collected at UHL and MMJ in summer 2019 (see Table S2 for specific dates). Five replicates of surface bog water were collected at each study site in June 2019. The depth of the water pools was less than 20 cm. The total number of water samples for δ15N analysis was 136.
A vertical peat core (10 cm in diameter and 30 cm deep) was collected in a Sphagnum-dominated lawn at each of the study sites in October 2018, kept vertically at 6 ∘C for 12 h, and then frozen. At the same time, 12 samples of living Sphagnum were collected randomly throughout each bog for species identification and N-isotope analysis. Additionally, 14 replicate samples of living Sphagnum were collected from various parts of each of the peat bogs for a 15N2-labeling experiment. Each replicate sample consisted of 30 individual 5 cm long Sphagnum plants. S. girgensohnii was used in the UHL and MMJ experiments, whereas a mix of S. magellanicum, S. papillosum, and S. cuspidatum was used in the BRU experiment (see Table S1). Sphagnum samples were transported to the laboratory at a temperature of 6 ∘C; transportation took 2–4 h. The wet samples were then kept at 6 ∘C until laboratory experiments started 2–6 d after moss collection. Prior to incubation, the moss samples were kept at 22 ∘C for 4 h.
In Sect. 3.3.3, we will compare the N-isotope composition of living Sphagnum and surface bog water. These two sample types were collected ca. 8 months apart. Previous research based on 210Pb peat core dating has indicated that 5 cm long Sphagnum capitula and stems at 14 rain-fed central European sites represent more than a 3-year growth increment (Novak et al., 2003, 2008). Hence, N-isotope comparisons of living Sphagnum and bog water sampled less than 1 year apart may still provide useful information.
2.3 15N2 Sphagnum incubation experiment
Measurements of potential N2-fixation rates were performed using a modified protocol following Larmola et al. (2014). Four plant replicates per site were analyzed at time t=0 without incubation (control no. 1). Ten replicates per site were closed in 200 mL transparent PE containers with 5 mL of bog water collected at BRU, UHL, and MMJ, respectively. Of these, four Sphagnum replicates with no 15N2 addition served as a procedural control (control no. 2) to identify possible incubation artifacts after 168 h. In the remaining six closed containers with Sphagnum replicates, 24 mL of headspace air was removed at t=0 and replaced with 32 mL of 15N2-tracer gas containing 98 at. % of 15N (Aldrich, Germany). Two 15N-labeled replicates were incubated for 48 h; another four 15N-labeled replicates were incubated for 168 h. According to Zivkovic et al. (2022), BNF rates peak in summer at relatively high ambient temperatures. We used data from the Czech Hydrometeorological Institute to set the incubation temperatures. The nearest high-elevation weather stations were Šerák (15 km from MMJ, 1328 m a.s.l., meters above sea level), with a daytime mean temperature for the 21 June–23 September 2017 period of 16.7 ∘C and a nighttime mean temperature of 9.4 ∘C, and Karlova Studánka (18 km from MMJ, 795 m a.s.l.), with analogous respective temperature averages of 16.2 and 11.5 ∘C. Each day, the temperature in the growth chamber in our experiment was kept at 18 ∘C for 16 h during daylight and at 10 ∘C for 8 h under dark conditions. The duration of daylight and darkness was unified with the experimental conditions applied by van den Elzen et al. (2017).
Following N-isotope analysis, BNF rates were calculated according to Vile et al. (2014) and Knorr et al. (2015):
where N2fix is the N2-fixation rate (in g N g DW−1(Sph) d−1, where DW represents dry weight), t is incubation time (in days), total N %Sph is the total amount of nitrogen (as a percentage), Δ at. % 15NSph is the difference between the at. % labeled and control sample, and Δ at. % 15Ngas is the difference between the concentration of 15N in the headspace and the natural abundance (at. %). The used Sphagnum density was 0.04 g cm−3.
We used larger sealed containers, compared with previous 15N2 experiments (that were shorter than ≤ 96 h; Myrold et al., 1999), to minimize the effect of changing headspace concentrations of O2 and CO2 on the living moss and the microbiome after 168 h. While, for example, van den Elzen et al. (2017) used 30 mL containers, Saiz et al. (2021) used 50 mL containers, and Stuart et al. (2021) worked with a container volume of 60 mL, we used a sealed 200 mL volume.
It bears mention that Dabundo et al. (2014) found a deviation from the declared 15N2 purity within commercially available tracer tanks. We did not study the tracer purity; hence, the observed BNF rates might be viewed as maximum estimates. Because our incubation study was based on one-time measurements under laboratory conditions, we chose not to upscale the BNF rates to the entire peat bog and an annual time span in the current paper.
2.4 Chemical and isotope analysis
Frozen peat cores were sectioned to 2 cm thick segments. Samples of peat and Sphagnum were dried and homogenized. Nitrogen concentrations in peat and Sphagnum samples were determined on a Fisons 1180 elemental analyzer with a 1.5 % reproducibility (2σ). Ammonium and nitrate concentrations in water samples were determined spectrophotometrically with a reproducibility of 0.1 mg L−1. About 0.5 L of each water sample was used to separate NH and NO (Bremner, 1965). Nitrogen-isotope composition was measured on a Delta V mass spectrometer and expressed in δ15N notation. International Atomic Energy Agency (IAEA) isotope standards N1 (δ15N = 0.4 ‰) and N2 (δ15N = 20.3 ‰) were analyzed before every session, and two in-house standards (ammonium sulfate, δ15N = −1.7 ‰, and glycine, δ15N = 4.0 ‰) were analyzed after every six samples. The reproducibility of the δ15N determinations was 0.30 ‰ and 0.35 ‰, for the liquid and solid samples, respectively. Methods of concentration analysis of other chemical species in the October 2018 samples are given in the Supplement.
2.5 Historical rates of Nr deposition
Long-term data from 32 monitoring stations in the Czech Republic operated by the Czech Hydrometeorological Institute, Prague, were used to assess the temporal and spatial variability in NH and NO concentrations in vertical deposition using a model by Oulehle et al. (2016). Median z-score values of NH and NO concentrations derived from observations at the monitoring stations and nation-wide emission rates, published by Kopacek and Vesely (2005) and Kopacek and Posh (2011), showed significant relationships at the p < 0.001 level. Using linear models, z-score values were expressed for the period from 1900 to 2012 and then back-transformed to give concentration estimates for the study sites. Annual rates of vertically deposited NH and NO were products of modeled concentrations and precipitation quantities at BRU, UHL, and MMJ.
2.6 Statistical evaluation
Statistical analysis was performed using the R software (R Core Team, 2019), version 3.6.2, and its contributed packages “sandwich” (Zeileis, 2004) and “multcomp” (Hothorn et al., 2008). Comparisons of groups of N-isotope and N-concentration data (see Sect. 2.3 and 2.4) were based on one-way analysis of variance with a sandwich estimator of covariance matrix to account for heteroscedasticity among the groups (MacKinnon and White, 1985). Post hoc multiple comparisons of the same groups were then performed according to Hothorn et al. (2008). Because of the largely uneven number of runoff samples per site (50, 6, and 2 at BRU, UHL, and MMJ, respectively), we did not include runoff δ15N data in the statistical evaluation.
3.1 Historical rates of atmospheric Nr inputs
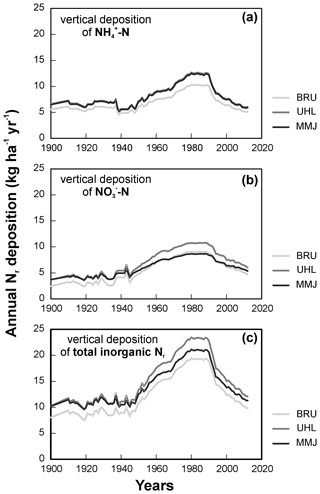
Vertical deposition of NH reached a maximum in 1980, remained almost unchanged until 1990, and decreased thereafter (Fig. 2a). Nitrate-N deposition exhibited a wider maximum between ca. 1970 and 1990 (Fig. 2b). In the oldest modeled time period (1900–1930), ammonium in deposition dominated over nitrate. During the deposition peak, the contributions of NH-N and NO-N to total vertical Nr deposition were similar (8 to 13 kg ha−1 yr−1 at individual sites). Across the modeled years, the NH-N NO-N ratio in vertical deposition was similar at all three sites (1.2–1.3; Table 1). Since ca. 1950, pollution at the study sites via total vertical deposition of inorganic Nr has increased in the following order: BRU < MMJ < UHL (Fig. 2c). Figure 2c shows that the between-site differences in the most recent years have been small (1–2 kg N ha−1 yr−1).
3.2 15N2 incubation experiment
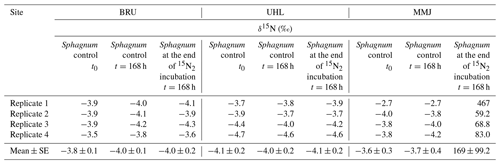
There was no change in the δ15N values of Sphagnum after 48 h at BRU and UHL (Fig. 3, Table 2). At MMJ, the average δ15N after 2 d increased to 3.0 ‰ (Fig. 3, Table 2). There were no statistically significant differences between δ15N values of Sphagnum at time t=0 and at time t=168 h following incubation in natural atmosphere (control nos. 1 and 2, p > 0.05; Table 2). Mean δ15N values of the moss of the two controls were similar among the sites (−3.6 ‰ to −4.1 ‰). At the end of the 168 h 15N2 Sphagnum incubation, there was still no change in the N-isotope signature of the moss at BRU and UHL (p > 0.05). In contrast, there was a large positive shift in δ15N values of Sphagnum collected at MMJ (59.2 ‰ to 467 ‰; Fig. 3, Table 2). The N2-fixation rate calculated from the N-isotope systematics in the 15N2-labeling experiment was 0 at BRU and UHL, whereas it was 4.11 µg N g−1 d−1 or 8.20 mg N m−2 d−1 at MMJ.
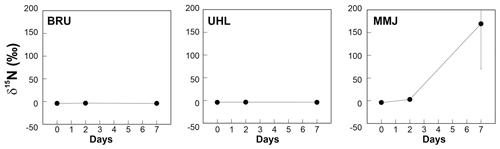
Figure 3Results of a 15N2 incubation study using living Sphagnum. Means and standard errors are given.
3.3 Natural-abundance N-isotope systematics
3.3.1 Atmospheric deposition
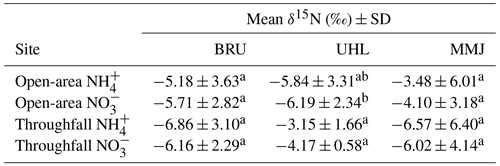
A total of 96 % of the deposited inorganic Nr species had negative δ15N values; i.e., contained isotopically light N (Fig. S1, Table S2). The mean δ15N value across all 181 samples of atmospheric deposition was −5.3 ± 0.3 ‰ (standard error – SE). Mean δ15N values of both forms of atmospherically deposited N (NH and NO) in an open area were slightly higher than those in throughfall at BRU and MMJ, whereas they were slightly lower than those in throughfall at UHL (Table 3). Nitrate-N in open-area deposition was, on average, slightly isotopically lighter than NH-N at all three sites. At the 0.05 probability level, however, the within-site differences among deposition sample types and among N species at BRU and MMJ were insignificant. The only statistically significant difference was found between δ15N values of open-area NO and both N species in throughfall at UHL (see superscript letters in Table 3).
3.3.2 Comparison of δ15N values of Sphagnum and atmospheric deposition
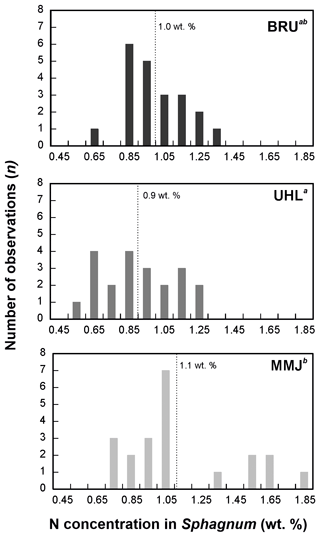
The δ15N values of living Sphagnum were between −6.2 ‰ and −1.9 ‰ (Table S1). The δ15N values of living Sphagnum at BRU were statistically different from the δ15N values of atmospheric deposition (means of −4.0 ‰ and −5.9 ‰, respectively; p < 0.05; Fig. 4). At UHL (means of −4.3 ‰ and −5.6 ‰, respectively;) and MMJ (means of −4.4 ‰ and −4.3 ‰, respectively), the differences between the δ15N values of living Sphagnum and the δ15N values of atmospheric deposition were insignificant (p > 0.05; Fig. 4). At BRU (and UHL), Sphagnum N was, on average, isotopically heavier than deposited N, i.e., closer to the 0 ‰ value of atmospheric N2. The nitrogen concentration in living Sphagnum was significantly higher at MMJ (mean of 1.1 wt %) than at UHL (0.9 wt %; p < 0.05; Fig. 5). The mean N concentration in BRU Sphagnum was 1.0 wt %, which is indistinguishable from the other two study sites.
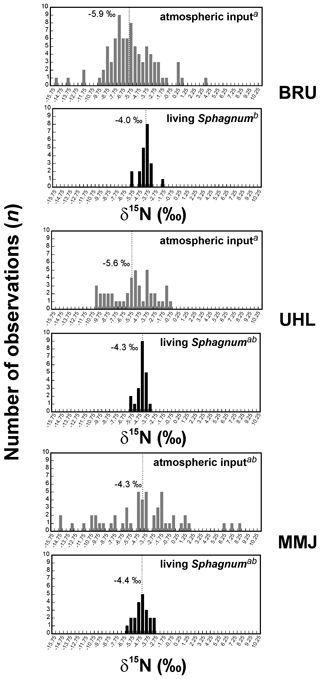
Figure 4Histograms of δ15N values of atmospheric input of Nr and living Sphagnum. Different letters in superscript mark statistically different sample types (p < 0.05).
3.3.3 Multiple δ15N comparisons among sample types
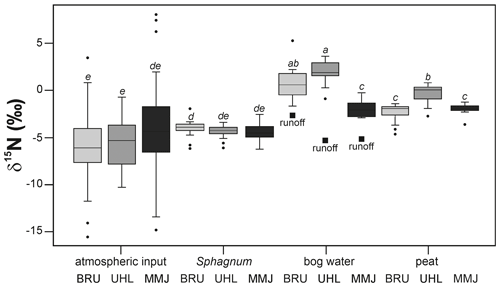
The mean δ15N value of surface bog water was 0.9 ‰ at BRU, 1.8 ‰ at UHL, and −1.9 ‰ at MMJ. Nitrogen in surface bog water was isotopically significantly heavier than N in both Sphagnum and atmospheric input at all three sites (p < 0.05; Fig. 6). At BRU and UHL, the mean δ15N value of surface bog water was higher than the 0 ‰ value of atmospheric N2. At MMJ, the mean δ15N value of surface bog water was lower than the N-isotope signature of atmospheric N2. In other words, all three sample types (deposition, Sphagnum, and bog water) at MMJ contained isotopically lighter N, compared with atmospheric N2 (Fig. 6).
When averaged across all depths (0–30 cm), the mean δ15N value in the peat core was −2.4 ‰ at BRU, −0.4 ‰ at UHL, and −1.9 ‰ at MMJ. At all three sites, the maturating peat in the vertical profile contained isotopically significantly heavier N compared with living Sphagnum (p < 0.05; Fig. 6, Table S2).
The mean δ15N value of runoff was −2.7 ‰ at BRU (combined NH and NO data; number of observations n=50), −5.3 ‰ at UHL (n=6), and −5.1 ‰ at MMJ (n=2; Table S1). The N-isotope signature of runoff was higher compared with the atmospheric input at BRU, whereas it was similar to the atmospheric input at UHL and MMJ (small, solid squares in Fig. 6). At all three sites, runoff contained isotopically lighter N compared with bog water (Fig. 6).
3.4 Chemistry of natural waters
3.4.1 Acidity
Surface bog water had a lower pH than atmospheric deposition and runoff at all three sites. The mean bog water pH was 4.0 at UHL, 4.3 at BRU, and 4.9 at MMJ (Table S3; data for October 2018). The pH of atmospheric deposition was only lower than 5.0 at the UHL site.
3.4.2 Nitrogen
The maximum NH-N concentration in open-area precipitation was 1.7 mg L−1 (UHL), and the maximum NO-N concentration in open-area precipitation was 7.1 mg L−1 (BRU; Table S2). The maximum concentration of NH-N in throughfall was 3.9 mg L−1(MMJ), and the maximum concentration of NO-N in throughfall was 9.7 mg L−1 (BRU; Table S2). The maximum concentration of NH-N in bog water was 2.3 mg L−1(UHL), and the maximum concentration of NO-N in bog water was 2.7 mg L−1 (MMJ; Table S2). The maximum concentration of NH-N in runoff was 1.3 mg L−1 (BRU), and the maximum concentration of NO-N in runoff was 7.1 mg L−1 (BRU; Table S2).
3.4.3 Phosphorus
The mean concentration of total P in atmospheric deposition increased in the following order: BRU (below 6.0 µg L−1) < UHL (9.3 µg L−1) < MMJ (15.5 µg L−1; Table S3; data for October 2018). Phosphorus concentration in surface bog water was roughly 30 times higher than in atmospheric deposition at BRU, more than 50 times higher at UHL, and more than 10 times higher at MMJ (Table S3). The UHL bog water contained as much as 490 µg P L−1. The mean P concentration in runoff increased in the following order: MMJ (12.4 µg L−1) < BRU (29.4 µg L−1) < UHL (40.2 µg L−1; Table S3).
3.4.4 Other chemical species
Natural waters at UHL were richer in sulfate (SO) than those at the remaining two sites (Table S3). UHL bog water and runoff contained as much as 47.4 and 33.7 mg SO L−1, respectively. Bog water was richer in potassium (K+) at UHL (9.05 mg L−1) compared with BRU and MMJ (1.85 and 1.97 mg L−1, respectively). The concentration of dissolved organic carbon (DOC) in atmospheric deposition was 2–4 times higher at MMJ than at the remaining two sites (Table S3). In contrast, surface bog water at MMJ had 1.4- to 2-fold lower DOC concentrations, compared with the remaining two sites. Detailed water chemistry for October 2018 is given in Table S3.
3.5 Vertical peat profiles
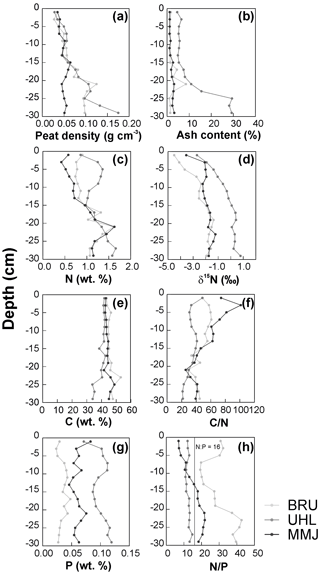
From the peat surface to the depth of 15 cm, peat density exhibited a slight increase that was similar at the three sites (Fig. 7a). Deeper, peat density remained relatively low (∼ 0.05 g cm−3) at MMJ, whereas it continued increasing irregularly at BRU and UHL. Ash content remained below 5 wt % to a depth of 30 cm at MMJ and, with one exception, also at BRU (Fig. 7b). The highest ash content was observed at UHL. Below a depth of 20 cm, it increased downcore to values greater than 10 wt %. The total N concentrations in peat substrate increased downcore or exhibited a zigzag pattern (Fig. 7c). The UHL peat core was the richest in N in most 2 cm peat sections. Down to a depth of 15 cm, the N concentration was the lowest in MMJ peat. This contrasts with the bottom panel of Fig. 5, which shows that the average N concentrations in replicated grab samples of living Sphagnum were relatively high. The apparent paradox, however, suffers from an uneven replication in Figs. 5 and 7 (n=21 and n=1, respectively). The N-concentration data in the MMJ peat core may just illustrate the large N-concentration variability at the moss surface. At all three sites, the vertical δ15N profile was characterized by a downcore increase near the surface, flattening out in the deepest peat sections (Fig. 7d). Generally, the δ15N values in peat cores increased in the following order: BRU < MMJ < UHL.
The nearly constant carbon (C) concentrations in peat were similar at all three sites to a depth of 20 cm and became more variable deeper (Fig. 7e). The sharpest downcore decrease in the C : N ratio was found at MMJ, with the exception of the 0–4 cm depth where the C : N ratio increased (Fig. 7f). Throughout the vertical peat profiles, the P concentration was the lowest at BRU and the highest at UHL (Fig. 7g). The N : P ratio was close to 12 throughout the UHL peat profile, increased downcore at MMJ from 10 to 20, and exhibited an irregular pattern at BRU, ranging between 20 and 40 (Fig. 7h). Further information on vertical changes in peat composition is given in Table S4.
4.1 The role of horizontal Nr deposition in peatlands
Using field experiments, we have recently shown a sizable contribution of horizontally deposited Nr to total atmospheric deposition in central European Sphagnum peat bogs (Novak et al., 2015b). During 80–90 d of the spring and fall foggy seasons, horizontal deposition added another 45 % to vertical deposition at Kunštátská kaple Bog (KB), a mountaintop site in the northern Czech Republic, and 14 % at Blatenska slat' (BS) in the less polluted southern Czech Republic (see Fig. 1 for location). Additionally, Hunova et al. (2023) reported a relatively high horizontal contribution of nitrate-N to winter-time atmospheric deposition in Czech mountains by analyzing ice accretions (mean of 29 ± 3 %; data for December–March; number of sites n=10). As a first approximation, we suggest that the upper limit of the contribution of horizontal deposition to vertical deposition at BRU, UHL, and MMJ could have been 30 %. If so, the total average Nr deposition was slightly higher than 18 kg ha−1 yr−1 at UHL and MMJ, whereas it was 16.5 kg ha−1 yr−1 at BRU (Table 1). Thus, our study sites can be considered to be highly or intermediately polluted (Lamers et al., 2000). The overall Nr pollution decreased in the following order: UHL > MMJ > BRU.
We note that total atmospheric deposition may also contain measurable amounts of total organic N (TON; Violaki et al., 2010; Cornell, 2011). TON fluxes have not been considered to be part of the Nr input in existing peatland BNF studies. Open-area precipitation at BRU, UHL, and MMJ contained an additional 15 %, 45 %, and 13 % of total organic N, respectively, relative to the sum of the two inorganic Nr forms (Table S3; October 2018). More TON data in precipitation would be needed to realistically estimate the annual deposition of organic N at our study sites.
4.2 Relationship between Nr pollution and N2 fixation
In theory, chronic atmospheric deposition of pollutant Nr should suppress BNF in peatlands (Wieder et al., 2019, 2020). Saiz et al. (2021) quantified downregulation of BNF along a geographical pollution gradient. Relative to a practically unpolluted site receiving 2 kg Nr ha−1 yr−1 from the atmosphere, these authors reported a 54 % decrease in BNF rates under an atmospheric deposition rate of 6 kg Nr ha−1 yr−1, a 69 % decrease under a deposition rate of 17 kg Nr ha−1 yr−1, and a 74 % decrease under a deposition rate of 27 kg Nr ha−1 yr−1. As seen in Fig. 3, our data did not confirm such an inverse correlation at central European sites. Instead, the most and least Nr-polluted peat bog exhibited no instantaneous BNF, whereas MMJ, whose Nr inputs were lower than those at UHL and higher than those at BRU, showed a high mean BNF rate. Given that most previous studies of Sphagnum bogs reported nonzero BNF rates regardless of atmospheric Nr deposition level (see compilation in Table S5), non-detectable BNF rates at BRU and UHL were surprising. The mean instantaneous BNF rate at MMJ was lower than BNF rates in unpolluted high-latitude bogs in Canada (Vile et al., 2014) and Patagonia (Knorr et al., 2015). Among the studies listed in Table S5, the mean BNF rates at MMJ were the fourth highest. Our data from MMJ are consistent with a conclusion by Saiz et al. (2021), who suggested the development of diazotrophic microbes' tolerance to high rates of atmospheric Nr deposition in recent decades. Global assessments of the dependence of BNF on total Nr deposition are difficult to make for several reasons: (i) few studies consider horizontal Nr deposition, which may be sizable and depends not just on atmospheric pollution but also on elevation, and few studies have quantified the atmospheric input of organic N; (ii) there is a large within-site heterogeneity in BNF (15N2 incubations should be performed using a large number of replicates; see δ15N differences between individual MMJ replicates in Table 2; see “BNF hotspots” in Stuart et al., 2021); and (iii) recalculation between two commonly used BNF units (µg N per 1 g of Sphagnum d−1 and g N m−2 yr−1) in literature data requires information on additional site-specific parameters, such as peat density, seasonality in daily temperatures, and snow cover duration. Additionally, it is often unclear to what maximum depth in peat bogs BNF proceeds and whether there is a gradient in BNF rates within this depth range (Vile et al., 2014; Knorr et al., 2015).
As the differences in Nr deposition among sites were minor (Fig. 2, Table 1), we suggest that Nr deposition was not the primary control on the BNF rates in our study at the time of Sphagnum sampling.
4.3 Chemical and environmental parameters as possible BNF controls
4.3.1 The role of the NH-N NO-N ratio in atmospheric deposition
The impact of the two main Nr forms in deposition on BNF can be different. Because BNF generates NH, the need for BNF to complement the metabolic demands of the moss may be lower if the deposition of NH-N exceeds the deposition of NO-N (van den Elzen et al., 2018; Saiz et al., 2021). At our study sites, the NH-N NO-N ratios were nearly identical (Table 1), slightly exceeding 1. It follows that this ratio was unlikely the driver of higher BNF potential at MMJ, compared with the remaining two sites.
4.3.2 The effect of temperature
MMJ is situated at a lower elevation, compared with UHL and BRU, and its mean annual temperature is higher than that at the remaining two sites (Table 1). This could positively affect the rate of BNF (Basilier et al., 1978; Schwintzer et al., 1983; Urban and Eisenreich, 1988; Zivkovic et al., 2022; Yin et al., 2022). By contrast, Carrell et al. (2019) argued that BNF rates may decrease with increasing temperature due to lower microbial diversity and greater mineralization rates, leading to more Nr in bog water and, hence, lower demand for BNF. Under the field conditions observed at the Czech sites and at the peatland scale, temperature is likely a key factor regulating BNF. In our 15N assimilation study, however, the chosen temperature was identical for all three sites. Consequently, temperature was not the dominant control on the measured short-term BNF rates.
4.3.3 The effect of bog wetness
Figure S2 shows monthly measurements of the water table level below the bog surface at BRU (Bohdalkova et al., 2013) and UHL (Tacheci, 2002). The mean annual water table depth was −5.2 ± 2.3 and −7.5 ± 1.1 cm at BRU at UHL, respectively. No water level monitoring data are available for MMJ; however, during our field sampling campaigns, numerous 10–20 cm deep water pools were observed near the bog center at MMJ, especially during the growing seasons of 2017 and 2019. Other high-elevation peat bogs on crystalline bedrock previously studied in the Czech Republic exhibited water table fluctuation at shallow depths of 5–8 cm, similar to BRU and MMJ (Novak and Pacherova, 2008). Based on visual inspection, somewhat drier conditions were typical of UHL, compared with the other two sites. Hydrological monitoring (GEOMON, GEOchemical MONitoring, network database, Czech Geological Survey; Oulehle et al., 2021b) revealed significantly drier conditions at UHL in the water year 2018, compared with the long-term average given in Table 1. Precipitation totals at UHL were 1460 mm in 2016, 1370 mm in 2017, a mere 892 mm in 2018, and 1230 mm in 2019. The ecosystem also suffered from chronic drought in 2018 at other GEOMON sites: JEZ (the nearest site to BRU) and UDL (the nearest site to MMJ; for location see Fig. 1). While Sphagnum for the 15N2 incubation was collected at all three study sites at the same time (October 2018), site-specific moisture conditions could have affected microbial community structure and the BNF potential. In the laboratory experiment, however, similar wetness was ensured by the same volume of added bog water to Sphagnum from all three sites. Therefore, we suggest that water availability did not control the instantaneous BNF rates.
4.3.4 The effect of Sphagnum species
Stuart et al. (2021) showed that host identity is often the primary driver of BNF in peatlands. Under low Nr pollution, higher species-specific litter decomposability augments BNF by increasing nutrient turnover (van den Elzen et al., 2020). Saiz et al. (2021) observed higher BNF rates in Sphagnum species typical of hollows compared with those dominating hummocks. Specifically, S. fallax exhibited higher BNF rates than S. capillifolium and S. papillosum. The reason for such systematics appeared to be that the anoxic environment of wet hollows is more favorable for N2 fixers (Leppanen et al., 2015; Zivkovic et al., 2022). By contrast, Vile et al. (2014) observed higher BNF rates in the hummock species S. fuscum than in the hollow-endemic species S. angustifolium. All moss samples for our 15N assimilation experiment were collected from lawns. One exception was a subordinated number of plants of S. cuspidatum , typical of hollows in the BRU incubation. While the moss species were identical in the UHL and MMJ incubation (S. girgensohnii), the BNF potential at these two sites was strikingly different (Fig. 3). Therefore, we suggest that Sphagnum species was not a key BNF control in our 15N2 experiment.
4.3.5 Organic-N availability
Wang et al. (2022) stressed the positive effect of organic N on BNF. The assimilation cost of amino acids was shown to be lower than that of NH (Liu et al., 2013; Song et al., 2016). Organic-N molecules can also serve as a C source for cyanobacteria, thus saving the cost of photosynthesis (Krausfeld et al., 2019). As seen in Table S3, concentrations of total organic N (TON) in bog water increased in the following order: MMJ < BRU < UHL. Thus, they were probably unrelated to augmented BNF at MMJ sensu Wang et al. (2022).
4.3.6 Possible P limitation
Phosphorus is needed for the synthesis of ATP, thereby playing a key role in symbiotic BNF (Rousk et al., 2017; Wieder et al., 2022). In plant tissues, N : P ratios greater than 16 may indicate P limitation, while N : P ratios lower than 16 correspond to N limitation (Koerselman and Meuleman, 1996). Caution must be exercised in interpreting N : P ratios in atmospheric deposition as potential controls of P or N limitation. In addition to atmospheric input fluxes, bioavailable P and N in bog waters are strongly affected by a tight inner cycling with additional inputs from biomass decomposition (Walbridge and Navaratnam, 2006). Phosphorus input fluxes via atmospheric deposition into peat bogs may affect nutrient limitation in the long run, depending on whether these input fluxes are large enough, compared with the frequently observed P leaching to deeper peat layers (Walbridge and Navaratnam, 2006, and references therein). According to Table S3, atmospheric deposition at all three study sites is consistent with P limitation that might limit BNF (high N : P ratios of 169, 60, and 112 at BRU, UHL, and MMJ, respectively). At the same time, N : P ratios in surface bog water were below 16 at two of the three sites: UHL (7.6) and MMJ (15). At BRU (N : P = 24). Phosphorus limitation inferred from bog water chemistry would provide an explanation for non-detectable instantaneous BNF. At UHL, we found no indication of a relationship between P availability and zero BNF. The relatively P-rich bog water (165–490 µg P L−1; Table S3) at all sites may contain, in addition to deposited P and mineralized P released during peat degradation, geogenic P. Bedrock granite (BRU and UHL) contains P in accessory apatite and K-feldspar, whose weathering was probably more intense during the recent 40 years of acid rain. Phosphorus in phyllite (MMJ) is concentrated in apatite. Phosphorus concentrations in fresh bedrock were similar at BRU and MMJ (52–55 ppb), whereas they were 2-fold lower at UHL (29 ppb; Gurtlerova et al., 1997; Pecina, 1999). The possible input of bioavailable geogenic P depended on local hydrology and could be site-specific.
Living Sphagnum had N : P ratios of 31, 12, and 7 at BRU, UHL, and MMJ, respectively (Table S4), indicating conditions favorable for BNF at the latter two sites. As seen in Fig. 7h, N : P < 16 (marking N limitation) was characteristic of the entire vertical peat profile at UHL as well as downcore to a depth of 15 cm at MMJ. In contrast, the N : P ratio was above 16 throughout the vertical peat profile at BRU. Phosphorus availability inferred from bog water and living Sphagnum gave consistent results with respect to possible BNF. As mentioned above, P likely limited BNF only at the BRU site.
Recently, measurements of regional P deposition started in headwater catchments of the GEOMON network (Oulehle et al., 2017). In the time period from 2014 to 2018, UHL, a site directly included in the GEOMON network, exhibited lower P concentrations in the atmospheric input, compared with JEZ in the west (a proxy for BRU) and UDL in the east (a proxy for MMJ; the distance between JEZ and UDL as well as between BRU and MMJ was approximately 70 km; see Fig. 1 for catchment locations). The 4-year average P concentrations at UHL were 72 and 36 µg L−1 in open-area precipitation and spruce throughfall, respectively. At JEZ, analogous P concentrations were 103 and 87 µg L−1. At UDL, these sample types contained on average of 110 and 91 µg P L−1, respectively. The high P uptake by the tree canopy resulting in a low P concentration in throughfall might indicate P deficiency in UHL inputs. At the same time, the N : P ratio in the total vertical atmospheric deposition was lower than 16 at all three sites (13.1 at JEZ, 15.5 at UHL, and 13.7 at UDL; GEOMON Hydrochemical Database, Czech Geological Survey).
4.3.7 Possible molybdenum (Mo) limitation
Nitrogenase requires molybdenum (Mo) in its active center to reduce N2 to bioavailable NH (Rousk et al., 2017; Bellenger et al., 2020). In principle, Mo limitation of BNF may have played a role in the contrasting BNF potentials observed at our sites. We do not have data on Mo concentrations in the studied ecosystems, except for trace-metal analysis of the prevailing rock types (≤ 1 ppm; Gurtlerova et al., 1997). However, known Mo contents in coal massively mined/burned in the central European industrial region could shed some light on Mo availability via atmospheric deposition: North Bohemian soft coal (Sokolov Basin close to BRU; Fig. 1) contains on average 18 ppm Mo, whereas Upper Silesian stone coal (Ostrava close to MMJ; Fig. 1) contains only ∼ 0.6 ppm Mo, i.e., 30 times less (Bouska et al., 1997). As UHL is situated downwind of the North Bohemian cluster of coal-burning power plants and very close to Turow (soft-coal mining in the Polish part of the Lusatian Neisse Basin; Fig. 1), atmospheric Mo inputs at UHL may be relatively high. It appears to be unlikely that Mo significantly influences the contrasting BNF potentials at our study sites.
4.3.8 The role of SO deposition
Large atmospheric inputs of acidifying sulfur forms (SO2 and H2SO4), characterizing the northern Czech Republic since the 1950s (Hunova et al., 2022), can affect BNF in two ways: by suppressing methanogenesis and by reducing the pH. Sulfate in peat bogs under high S deposition becomes an important electron acceptor (Pester et al., 2012), and bacterial sulfate reduction is thermodynamically favored relative to methanogenesis and fermentative processes (Vile et al., 2003). It not only decreases gross CH4 production in peat, mitigating the flux of CH4 to the atmosphere and minimizing climate warming, but also lowers the supply of CH4 to methanotrophs that, at some sites, represent a major BNF pathway (Dise and Verry, 2001; Vile et al., 2014). Large SO inputs may, thus, suppress BNF in peat bogs. In this context, is should also be mentioned that a 34S 32S isotope study has documented post-depositional vertical mobility of S in industrially polluted peat bogs (Novak et al., 2009). While long-term vertical S deposition, calculated according to Oulehle et al. (2016), was similarly high at UHL and MMJ (respective means of 18.6 and 17.0 kg ha−1 yr−1 for the 1900–2012 period), higher than at BRU (12.2 kg ha−1 yr−1), UHL bog water at the time of this study was nearly 70 times richer in SO than MMJ bog water and 8 times richer in SO than BRU bog water (Table S3). Runoff at UHL was 4–5 times richer in SO than runoff at MMJ and BRU. The zero instantaneous BNF at UHL in our 15N2 incubation could be related to the highly elevated S deposition in the case that UHL primarily hosts methane oxidizing diazotrophs.
UHL waters were characterized by lower pH, compared with those at MMJ and BRU (Table S3). Runoff pH at UHL was 4.48, while runoff pH at MMJ was 7.40. Bog water pH at UHL was 4.02, while pH at MMJ was 4.88. Downregulation of BNF in a more acidic environment has been reported, e.g., by Basilier (1979) and van den Elzen et al. (2017). Accordingly, the lack of BNF at UHL may be related to its lower pH, compared with the other two study sites.
4.4 Natural-abundance N-isotope systematics
Sphagnum metabolizes bioavailable NH approximately 8 times faster than NO (Saiz et al., 2021). Because there were nonsignificant differences between δ15N values of NH and NO in rainfall at our study sites (Fig. S1), it is reasonable to use the entire δ15N data set for a comparison with δ15N values of living Sphagnum (Fig. 4). Slow lateral mixing of surface bog waters may bring throughfall N from the forested margins of each bog to the central unforested area; therefore, we additionally included throughfall δ15N data in Fig. 4 comparisons. The isotopically analyzed living Sphagnum plants represented a more than 3-year increment (see Sect. 2.2). We found a statistically significant shift from isotopically light N of the deposition to isotopically heavier N of Sphagnum only at BRU (p < 0.05). This might indicate mixing with even heavier atmospheric N2 taken up by diazotrophs. At BRU, BNF might have intermittently proceeded over the most recent growing seasons, even though the 15N2 experiment did not corroborate this process in October 2018.
A straightforward attribution of the N-isotope pattern at BRU to BNF, however, is hampered by the fact that mineralization is a likely alternate source of dissolved Nr for assimilation by the moss (Zivkovic et al., 2022, and references therein). The often found high δ15N values of mineralized Nr remaining in the bog ecosystem result from isotope fractionation accompanying denitrification, a process known to occur especially in peat bogs that are not extremely acidic. Gaseous products of denitrification contain isotopically light N both in wetlands (Denk et al., 2017; for data from Czech peat bogs, see Novak and al., 2015a, 2018) and aerated forest soils (Houlton and Bai, 2009; for data from Czech upland soils, see Oulehle et al., 2021a). Nitrogen in surface bog water at BRU had a positive mean δ15N value of 0.9 ‰ (Fig. 6). Thus, isotope systematics at BRU are consistent with the incorporation of mineralized Nr into moss biomass during assimilation instead of uptake of N resulting from BNF.
Advancing mineralization accompanying peat maturation with mobilization and export of gaseous low-δ15N nitrogen is also responsible for the increasing δ15N values of the residual peat substrate downcore (Fig. 7d).
Figure S3 summarizes two general scenarios under which a difference between the N-isotope composition of atmospheric input, Sphagnum, and bog water indicates BNF. In the first scenario, the mean δ15N values increase in the following order: deposited Nr < bog water Nr < Sphagnum Nr < atmospheric N2. In the second scenario, the mean δ15N values decrease in the following order: deposited Nr > bog water Nr > Sphagnum Nr > atmospheric N2. Whereas the δ15N value of bulk atmospheric deposition in central Europe is mostly negative, positive mean δ15N values have been reported from other regions. One example is isotopically heavy N of dry-deposited HNO3 in an industrial part of the USA (Elliott et al., 2009). Figure S3 assumes that the magnitude of potential N-isotope fractionations during the uptake of inorganic N into plant biomass is relatively small and does not overprint the larger N-isotope differences between the abovementioned mixing end-members.
It remains to be seen how to reconcile the relatively high instantaneous BNF rate at MMJ, measured in the laboratory, with the nonexistence of a positive δ15N shift from atmospheric deposition (mean of −4.3 ‰) to Sphagnum (mean of −4.4 ‰; p > 0.05; Fig. 4). Given that we explained the positive δ15N shift from deposition to Sphagnum at BRU by mixing of low-δ15N rainfall with high-δ15N bog water and that bog water N at MMJ is isotopically heavy, a similar positive N-isotope shift from rainfall to Sphagnum would also be expected at MMJ. Such was not the case. This observation is important because it might indicate that the uptake of recently mineralized Nr from bog water at sites hydrologically similar to MMJ (and also BRU) may not control the N-isotope signature of living Sphagnum. An input of isotopically light Nr for assimilation by the MMJ moss could, in principle, originate from shallow groundwater upwelling or lateral water inflow from other segments of the catchment possibly bringing legacy low-δ15N nitrogen from the peak acid rain period throughfall. Such within-site water inputs could affect the intermediate δ15N value of Sphagnum at MMJ.
Based on hydrochemical monitoring data and statistical modeling, the three studied Sphagnum peat bogs located in the industrial northern Czech Republic received close to 18 kg Nr ha−1 yr−1 via atmospheric deposition. Since 1900, the level of the atmospheric input of Nr affected the study sites in the following order: UHL > MMJ > BRU. In the most recent years, the annual Nr inputs via vertical deposition between the sites differed by a mere 1 to 2 kg ha−1 yr−1. Thus, the sites can be classified as highly to intermediately polluted. A 168 h 15N2 assimilation experiment revealed relatively high but variable rates of BNF at MMJ and non-detectable BNF at the remaining two sites, which were characterized by slightly higher and slightly lower Nr depositions compared with MMJ, respectively. We investigated 10 different parameters that might have served as controls on the presence or absence of instantaneous BNF in living moss. In addition to bulk Nr deposition fluxes, these parameters included the NH-N NO-N ratio in atmospheric input, temperature, wetness, Sphagnum species, organic-N availability, possible P limitation, possible Mo limitation, SO deposition, and pH. Using the available data, we argue that P deficiency was the likely inhibitor of BNF at BRU. Assuming that methanotrophic bacteria represented a major type of diazotrophs, extremely high SO inputs may have been the key control on the absence of BNF at UHL. While the long-term temperature and wetness at UHL were also lower, compared with the remaining two sites, they probably did not affect the results of the 15N2 experiment, as the incubation was performed under the same temperature and wetness conditions for all sites. In general, higher concentrations of decomposition-inhibiting metabolites could be causally related to BNF rates. However, such a control on BNF was unlikely because the same Sphagnum species from MMJ and UHL was used for the 15N2 experiment that showed contrasting results for these two sites. The large δ15N differences between moss replicates that were collected from various segments of MMJ at the end of the 15N2 incubation suggested the existence of BNF hotspots.
The use of natural-abundance N-isotope ratios to corroborate the observed instantaneous BNF rates was hampered by the isotopically heavy N of surface bog water. The bog water contained secondary Nr forms which could have resulted from partial Sphagnum/peat decomposition and removal of the complementary low-δ15N products of denitrification. At BRU, we found statistically significant differences in δ15N values in the following order: deposited Nr < Sphagnum Nr < atmospheric N2 < bog water Nr. Stable isotope ratios could not unambiguously distinguish between the assimilation of bog water Nr and atmospheric N2 to form the observed N-isotope signature of Sphagnum. At UHL and MMJ, δ15N differences between Sphagnum and the atmospheric input were statistically insignificant. The natural-abundance approach as a test of BNF presence may give more promising results at high-latitude sites often characterized by a greater (30–40 cm) depth of the water table level below Sphagnum capitula than the central European sites.
All data are presented in the paper and Supplement.
The supplement related to this article is available online at: https://doi.org/10.5194/soil-9-623-2023-supplement.
MS: conceptualization, data curation, visualization, and writing – review and editing; MN: conceptualization, data interpretation, and writing – original draft; BC: methodology, nitrogen fixation data acquisition, and data interpretation; IJ: methodology and concentration and isotope data acquisition; FB: methodology, data interpretation, and validation; FV: fieldwork; JC: fieldwork; EP: formal analysis and resources; AK: statistical analysis; LB: data interpretation.
The contact author has declared that none of the authors has any competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
This is a contribution to the Strategic Research Plan of the Czech Geological Survey (DKRVO/CGS 2018–2022; grant. no. 310660 to Marketa Stepanova). The authors wish to thank Martin Sanda (Czech Polytechnic, Prague) and Jan Knotek (Jeseník branch of the Czech Geological Survey) for fieldwork assistance. We are also grateful to Filip Oulehle for modeling long-term atmospheric N deposition at the study sites and to Oldrich Myska for providing monitoring data from the GEOMON database.
This research has been supported by the Česká geologická služba (grant no. 310660).
This paper was edited by Kate Buckeridge and reviewed by two anonymous referees.
Basilier, K.: Moss-associated nitrogen fixation in some mire and coniferous forest environments around Uppsala, Sweden, Lindbergia, 5, 84–88, 1979.
Basilier, K., Granhall, U., Stenström, T.-A., and Stenstrom, T.-A.: Nitrogen fixation in wet minerotrophic moss communities of a subarctic mire, Oikos, 31, 236–246, https://doi.org/10.2307/3543568, 1978.
Bellenger, J. P., Darnajoux, R., Zhang, X., and Kraepiel, A. M. L.: Biological nitrogen fixation by alternative nitrogenases in terrestrial ecosystems: A review, Biogeochemistry, 149, 53–73, https://doi.org/10.1007/s10533-020-00666-7, 2020.
Bohdalkova, L., Curik, J., Kubena, A. A., and Buzek, F.: Dynamics of methane fluxes from two peat bogs in the Ore Mountains, Czech Republic, Plant Soil Environ., 59, 14–21, https://doi.org/10.17221/330/2012-PSE, 2013.
Bohdalkova, L., Novak, M., Stepanova, M., Fottova, D., Chrastny, V., Mikova, J., and Kubena, A. A.: The fate of atmospherically derived Pb in Central European catchments: Insights from spatial and temporal pollution gradients and Pb isotope ratios, Environ. Sci. Technol., 48, 4336–4343, https://doi.org/10.1021/es500393z, 2014.
Bouska, V., Pesek, J., Kaigl, J., and Peskova, J.: Evaluation of engineering-geology conditions in the Sokolov Coal Basin, Contract Report, Faculty of Science, Charles University, Prague, 1997.
Bremner, J. M.: Inorganic forms of nitrogen, in: Methods of soil analysis, Part 2, Agronomy Vol. 9., edited by: Black, C. A., American Society of Agronomy, Madison, WI, 179–1237, ISBN: 9780891182047, 1965.
Buzek, F., Novak, M., Cejkova, B., Jackova, I., Curik, J., Veselovsky, F., Stepanova, M., Prechova, E., and Bohdalkova, L.: Assessing DOC export from a Sphagnum-dominated peatland using δ13C and δ18O-H2O stable isotopes, Hydrol. Process., 33, 2792–2803, https://doi.org/10.1002/hyp.13528, 2019.
Buzek, F., Cejkova, B., Jackova, I., Kram, P., Oulehle, F., Myska, O., Curik, J., Veselovsky, F., and Novak, M.: 15N study of the reactivity of atmospheric nitrogen in four mountain forest catchments (Czech Republic, Central Europe), Appl. Geochem., 116, 104567, https://doi.org/10.1016/j.apgeochem.2020.104567, 2020.
Carrell, A. A., Kolton, M., Warren, M. J., Kostka, J. E., Weston, D. J., and Kostka, J. E.: Experimental warming alters the community composition, diversity, and N2 fixation activity of peat moss (Sphagnum fallax) microbiomes, Glob. Change Biol., 25, 2993–3004, https://doi.org/10.1111/gcb.14715, 2019.
Chiewattanakul, M., McAleer, A. D., Reay, M. K., Griffiths, R. I., Buss, H. L., and Evershed, R. P.: Compound-specific amino acid 15N-stable isotope probing for the quantification of biological nitrogen fixation in soils, Soil Biol. Biochem., 169, 108654, https://doi.org/10.1016/j.soilbio.2022.108654, 2022.
Cornell, S. E.: Atmospheric nitrogen deposition: Revisiting the question of the importance of the organic component, Environ. Pollut., 159, 2214–2222, https://doi.org/10.1016/j.envpol.2010.11.014, 2011.
Czech hydrometeorological institute Annual Report, Hydrometeorological Institute, 1998.
Dabundo, R., Lehmann, M. F., Treibergs, L., Tobias, C. R., Altabet, M. A., Moisander, P. H., and Granger, J.: The contamination of commercial 15N2 gas stocks with 15N–labeled nitrate and ammonium and consequences for nitrogen fixation measurements, PloS One, 9, e110335, https://doi.org/10.1371/journal.pone.0110335, 2014.
Davies-Barnard, T. and Friedlingstein, P.: The global distribution of biological nitrogen fixation in terrestrial natural ecosystems, Global Biogeochem. Cy., 34, e2019GB006387, https://doi.org/10.1029/2019GB006387, 2020.
DeLuca, T. H., Zackrisson, O., Nilsson, M. C., and Sellstedt, A.: Quantifying nitrogen-fixation in feather moss carpets of boreal forests, Nature, 419, 917–920, https://doi.org/10.1038/nature01051, 2002.
Denk, T. R., Mohn, J., Decock, C., Lewicka-Szczebak, D., Harris, E., Butterbach-Bahl, K., Kiese, R., and Wolf, B.: The nitrogen cycle: A review of isotope effects and isotope modeling approaches, Soil Biol. Biochem. 105, 121–137, https://doi.org/10.1016/j.soilbio.2016.11.015, 2017.
Diakova, K., Biasi, C., Capek, P., Martikainen, P. J., Marushchak, M. E., Patova, E. N., and Santruckova, H.: Variation in N2 fixation in subarctic tundra in relation to landscape position and nitrogen pools and fluxes, Arct. Antarct. Alp. Res., 48, 111–125, https://doi.org/10.1657/AAAR0014-064, 2016.
Dise, N. B. and Verry, E. S.: Suppression of peatland methane emission by cumulative sulfate deposition in simulated acid rain, Biogeochemistry, 53, 143–160, https://doi.org/10.1023/A:1010774610050, 2001.
Dohnal, Z., Kunst, M., Mejstrik, V., Raucina, S., and Vydra, V.: Czechoslovak Peatlands, Czechoslovak Academy of Sciences, Czechoslovakia, 1965.
Elliott, E. M., Kendall, C., Boyer, E. W., Burns, D. A., Lear, G. G., Golden, H. E., Harlin, K., Bytnerowicz, A., Butler, T. J., and Glatz, R.: Dual nitrate isotopes in dry deposition: Utility for partitioning NOx source contributions to landscape nitrogen deposition, J. Geophys. Res., 114, G04020, https://doi.org/10.1029/2008JG000889, 2009.
Fottova, D. and Skorepova, I.: Changes in mass element fluxes and their importance for critical loads: GEOMON network, Czech Republic, Water Air Soil Pollut., 105, 365–376, https://doi.org/10.1007/978-94-017-0906-4_33, 1998.
Fritz, C., Lamers, L. P. M., Riaz, M., van den Berg, L. J. L., and Elzenga, T. J. T. M.: Sphagnum mosses – masters of efficient N-uptake while avoiding intoxication, PLoS ONE, 9, 1–11, https://doi.org/10.1371/journal.pone.0079991, 2014.
Frolking, S., Talbot, J., Jones, M. C., Treat, C. C., Kauffman, J. B., Tuittila, E. S., and Roulet, N.: Peatlands in the Earth's 21st century climate system, Environ. Rev., 19, 371–396, https://doi.org/10.1139/a11-014, 2011.
Gallego-Sala, A. V., Charman, D. J., Brewer, S., et al.: Latitudinal limits to the predicted increase of the peatland carbon sink with warming, Nat. Clim. Change, 8, 907–913, https://doi.org/10.1038/s41558-018-0271-1, 2018.
Gurtlerova, P., Dusek, P., and Fikr, S.: Regional Lithogeochemical Database, Czech Geological Survey, Prague, 1997.
Hemond, H. F.: The nitrogen budget of Thoreau's bog, Ecology, 64, 99–109, https://doi.org/10.2307/1937333, 1983.
Ho, A. and Bodelier, P. L.: Diazotrophic methanotrophs in peatlands: The missing link?, Plant Soil, 389, 419–423, https://doi.org/10.1007/s11104-015-2393-9, 2015.
Holland-Moritz, H., Stuart, J. E., Lewis, L. R., Miller, S. N., Mack, M. C., Ponciano, J. M., McDaniel, S. F., and Fierer, N.: The bacterial communities of Alaskan mosses and their contributions to N2-fixation, Microbiome, 9, 1–14, https://doi.org/10.1186/s40168-021-01001-4, 2021.
Hothorn, T., Bretz, F., and Westfall, P.: Simultaneous inference in general parametric models, Biometrical J., 50, 346–363, https://doi.org/10.1002/bimj.200810425, 2008.
Houlton, B. Z. and Bai, E.: Imprint of denitrifying bacteria on the global terrestrial biosphere, P. Nat. Acad. Sci. USA, 106, 21713–21716, https://doi.org/10.1073/pnas.0912111106, 2009.
Hunova, I., Novak, M., Kurfurst, P., Skachova, H., Stepanova, M., Prechova, E., Komarek, A., Curik, J., Veselovsky, F., and Bohdalkova, L.: Contribution of rime to atmospheric sulphur deposition in Central Europe: A combined empirical and modelling approach, Atmos. Environ., 270, 118877, https://doi.org/10.1016/j.atmosenv.2021.118877, 2022.
Hunova, I., Novak, M., Kurfurst, P., Skachova, H., Stepanova, M., Prechova, E., Veselovsky, F., Curik, J., Bohdalkova, L., and Komarek, A.: Comparison of vertical and horizontal atmospheric deposition of nitrate at Central European mountain-top sites during three consecutive winters, Sci. Total Environ., 869, 161697, https://doi.org/10.1016/j.scitotenv.2023.161697, 2023.
Knorr, K.-H., Horn, M. A., and Borken, W.: Significant nonsymbiotic nitrogen fixation in Patagonian ombrotrophic bogs, Glob. Change Biol., 21, 2357–2365, https://doi.org/10.1111/gcb.12849, 2015.
Koerselman, W. and Meuleman, A. F.: The vegetation N: P ratio: A new tool to detect the nature of nutrient limitation, J. Appl. Ecol., 33, 1441–1450, https://doi.org/10.2307/2404783, 1996.
Kolton, M., Weston, D. J., Mayali, X., Weber, P. K., McFarlane, K. J., Pett-Ridge, J., Somoza, M. M., and Kostka, J. E.: Defining the Sphagnum core microbiome across the North American continent reveals a central role for diazotrophic methanotrophs in the nitrogen and carbon cycles of boreal peatland ecosystems, MBio, 13, e03714-21, https://doi.org/10.1128/mbio.03714-21, 2022.
Kopacek, J. and Posch, M.: Anthropogenic nitrogen emissions during the Holocene and their possible effects on remote ecosystems, Global Biogeochem. Cy., 25, GB2017, https://doi.org/10.1029/2010GB003779, 2011.
Kopacek, J. and Vesely, J.: Sulfur and nitrogen emissions in the Czech Republic and Slovakia from 1850 till 2000, Atmos. Environ., 39, 2179–2188, https://doi.org/10.1016/j.atmosenv.2005.01.002, 2005.
Kox, M. A., Aalto, S. L., Penttilä, T., Ettwig, K. F., Jetten, M. S., and van Kessel, M. A.: The influence of oxygen and methane on nitrogen fixation in subarctic Sphagnum mosses, Amb. Express, 8, 1–9, https://doi.org/10.1186/s13568-018-0607-2, 2018.
Kox, M. A., van den Elzen, E., Lamers, L. P., Jetten, M. S., and van Kessel, M. A.: Microbial nitrogen fixation and methane oxidation are strongly enhanced by light in Sphagnum mosses, Amb. Express, 10, 1–11, https://doi.org/10.1186/s13568-020-00994-9, 2020.
Krausfeldt, L. E., Farmer, A. T., Castro Gonzalez, H. F., Zepernick, B. N., Campagna, S. R., and Wilhelm, S. W.: Urea is both a carbon and nitrogen source for Microcystis aeruginosa: Tracking 13C incorporation at bloom pH conditions, Front. Microbiol., 10, 1064, https://doi.org/10.3389/fmicb.2019.01064, 2019.
Lamers, L. P. M., Bobbing, R., and Roelofs, J. G. M.: Natural nitrogen filter fails in polluted raised bogs, Glob. Change Biol., 6, 583–586, https://doi.org/10.1046/j.1365-2486.2000.00342.x, 2000.
Larmola, T., Leppanen, S. M., Tuittila, E. S., Aarva, M., Merila, P., Fritze, H., and Tiirola, M.: Methanotrophy induces nitrogen fixation during peatland development, P. Nat. Acad. Sci. USA, 111, 734–739, https://doi.org/10.1073/pnas.1314284111, 2014.
LeBauer, D. S. and Treseder, K. K.: Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed, Ecology, 89, 371–379, https://doi.org/10.1890/06-2057.1, 2008.
Leppanen, S. M., Rissanen, A. J., and Tiirola, M.: Nitrogen fixation in Sphagnum mosses is affected by moss species and water table level, Plant Soil, 389, 185–196, https://doi.org/10.1007/s11104-014-2356-6, 2015.
Limpens, J., Berendse, F., and Klees, H.: How phosphorus availability affects the impact of nitrogen deposition on Sphagnum and vascular plants in bogs, Ecosystems, 7, 793–804, https://doi.org/10.1007/s10021-004-0274-9, 2004.
Limpens, J., Heijmans, M. P. D., and Berendse, F.: The nitrogen cycle in boreal peatlands, in: Boreal Peatland Ecosystems, edited by: Wieder, R. K. and Witt, D. H., Springer Berlin Heidelberg New York, 195–230, ISBN-13 978-3-540-31912-2, 2006.
Liu, X. Y., Koba, K., Makabe, A., Li, X. D., Yoh, M., and Liu, C. Q.: Ammonium first: Natural mosses prefer atmospheric ammonium but vary utilization of dissolved organic nitrogen depending on habitat and nitrogen deposition, New Phytol., 199, 407–419, https://doi.org/10.1111/nph.12284, 2013.
MacKinnon, J. G. and White, H.: Some heteroskedasticity-consistent covariance matrix estimators with improved finite sample properties, J. Econom., 29, 305–325, https://doi.org/10.1016/0304-4076(85)90158-7, 1985.
Marx, A., Hintze, S., Sanda, M., Jankovec, J., Oulehle, F., Dusek, J., Vitvar, T., Vogel, T., van Geldern, R., and Barth, J. A. C.: Acid rain footprint three decades after peak deposition: Long-term recovery from pollutant sulphate in the Uhlirska catchment (Czech Republic), Sci. Total Environ., 598, 1037–1049, https://doi.org/10.1016/j.scitotenv.2017.04.109, 2017.
Myrold, D. D., Ruess, R. W., and Klug, M. J.: Dinitrogen fixation, in: Denitrification, Standard soil methods for long-term ecological research, edited by: Robertson, G. P., Coleman, D. C., Bledsoe, C. S., nd Sollins, P., Oxford University Press, 241–257, ISBN: 9780195120837, 1999.
Novak, M. and Pacherova, P.: Mobility of trace metals in pore waters of two central European peat bogs, Sci. Total Environ., 394, 331–337, https://doi.org/10.1016/j.scitotenv.2008.01.036, 2008.
Novak, M., Emmanuel, S., Vile, M. A., Erel, Y., Veron, A., Paces, T., Wieder, R. K., Vanecek, M., Stepanova, M., Brizova, E., and Hovorka, J.: Origin of lead in eight Central European peat bogs determined from isotope ratios, strengths and operation times of regional pollution sources, Environ. Sci. Technol., 37, 437–445, https://doi.org/10.1021/es0200387, 2003.
Novak, M., Kirchner, J. W., Fottova, D., Prechova, E, Jackova, I., Kram, P., and Hruska, J.: Isotopic evidence for processes of sulfur retention/release in 13 forested catchments spanning a strong pollution gradient (Czech Republic, central Europe), Glob. Biogeochem. Cy., 19, GB4012, https://doi.org/10.1029/2004GB002396, 2005.
Novak, M., Brizova, E., Adamova, M., Erbanova, L., and Bottrell, S. H.: Accumulation of organic carbon over the past 150 years in five freshwater peatlands in western and central Europe, Sci. Total Environ., 390, 426–436, https://doi.org/10.1016/j.scitotenv.2007.10.011, 2008.
Novak, M., Zemanova, L., Jackova, I., Buzek, F., and Adamova, M.: Isotope composition of bulk carbon in replicated Sphagnum peat cores from three Central European high-elevation wetlands, Geochem. J., 43, 5–9, https://doi.org/10.2343/geochemj.1.0026, 2009.
Novak, M., Stepanova, M., Jackova, I., Vile, M. A., Wieder, R. K., and Buzek, F.: Isotopic evidence for nitrogen mobility in peat bogs, Geochim. Cosmochim. Ac., 123, 74–92, https://doi.org/10.1016/j.gca.2014.02.021, 2014.
Novak, M., Gebauer, G., Thoma, M., Curik, J., Stepanova, M., Jackova, I., Buzek, F., Barta, J., Santruckova, H., Fottova, D., and Kubena, A. A.: Denitrification at two N-polluted, ombrotrophic Sphagnum bogs in Central Europe: Insights from porewater N2O-isotope profiles, Soil Biol. Biogeochem., 81, 48–57, https://doi.org/10.1016/j.soilbio.2014.10.021, 2015a.
Novak, M., Veselovsky, F., Curik, J., Stepanova, M., Fottova, D., Prechova, E., and Myska, O.: Nitrogen input into Sphagnum bogs via horizontal deposition: An estimate for N-polluted high-elevation sites, Biogeochemistry, 123, 307–312, https://doi.org/10.1007/s10533-015-0076-5, 2015b.
Novak, M., Jackova, I., Curik, J., Stepanova, M., Veselovsky, F., Buzek, F., Vile, M. A., Bufkova, I., Valkova, I., Adamova, M., Bohdalkova, L., and Komarek, A.: Contrasting δ15N values of atmospheric deposition and Sphagnum peat bogs: N fixation as a possible cause, Ecosystems, 19, 1037–1050, https://doi.org/10.1007/s10021-016-9985-y, 2016.
Novak, M., Suarez, S. P., Gebauer, G., Thoma, M., Buzek, F., Cejkova, B., Jackova, I., Stepanova, M., Prechova, E., Curik, J., Veselovsky, F., Valkova, I., Blaha, V., Fottova, D., and Komarek, A.: Relationship between nitrogen isotope ratios of NO and N2O in vertical porewater profiles through a polluted rain-fed peat bog, Soil Biol. Biochem., 123, 7–9, https://doi.org/10.1016/j.soilbio.2018.04.022, 2018.
Novak, M., Pacherova, P., Elliott, E. M., Jackova, I., Stepanova, M., Curik, J., Cejkova, B., Buzek, F., Prechova, E., and Valkova, I.: δ15N systematics in two minerotrophic peatlands in the eastern US: Insights into nitrogen cycling under moderate pollution, Global Ecol. Conserv., 17, e00571, https://doi.org/10.1016/j.gecco.2019.e00571, 2019.
Oulehle, F., Kopacek, J., Chuman, T., Cernohous, V., Hunova, I., Hruska, J., Kram, P., Lachmanova, Z., Navratil, T., Stepanek, P., Tesar, M., and Evans, C. D.: Predicting sulphur and nitrogen deposition using a simple statistical method, Atmos. Environ., 140, 456–468, https://doi.org/10.1016/j.atmosenv.2016.06.028, 2016.
Oulehle, F., Chuman, T., Hruska, J., Kram, P., McDowell, W. H., Myska, O., Navratil, T., and Tesar, M.: Recovery from acidification alters concentrations and fluxes of solutes from Czech catchments, Biogeochemistry, 132, 251–272, https://doi.org/10.1007/s10533-017-0298-9, 2017.
Oulehle, F., Fischer, M., Hruska, J., Chuman, T., Kram, P., Navratil, T., Tesar, M., and Trnka, M.: The GEOMON network of Czech catchments provides long-term insights into altered forest biogeochemistry: From acid atmospheric deposition to climate change, Hydrol. Process., 35, e14204, https://doi.org/10.1002/hyp.14204, 2021a.
Oulehle, F., Goodale, C. L., Evans, C. D., Chuman, T., Hruska, J., Kram, P., Navratil, T., Tesar, M., Ac, A., Urban, O., and Tahovska, K.: Dissolved and gaseous nitrogen losses in forests controlled by soil nutrient stoichiometry, Environ. Res. Lett., 16, 064025, https://doi.org/10.1088/1748-9326/ac007b, 2021b.
Pecina, V.: Regional Lithogeochemical Database, Czech Geological Survey, Prague, 1999.
Pester, M., Knorr, K. H., Friedrich, M. W., Wagner, M., and Loy, A.: Sulfate-reducing microorganisms in wetlands – fameless actors in carbon cycling and climate change, Front. Microbiol., 3, 1–19, https://doi.org/10.3389/fmicb.2012.00072, 2012.
R Core Team: R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org (last access: 20 December 2019), 2019.
Rousk, K., Jones, D. L., and DeLuca, T. H.: Moss-cyanobacteria associations as biogenic sources of nitrogen in boreal forest ecosystems, Front. Microbiol., 4, 1–10, https://doi.org/10.3389/fmicb.2013.00150, 2013.
Rousk, K., Sorensen, P. L., Lett, S., and Michelsen, A.: Across-habitat comparison of diazotroph activity in the subarctic, Microbial Ecol., 69, 778–787, https://doi.org/10.1007/s00248-014-0534-y, 2015.
Rousk, K., Degboe, J., Michelsen, A., Bradley, R., and Bellenger, J. P.: Molybdenum and phosphorus limitation of moss-associated nitrogen fixation in boreal ecosystems, New Phytol., 214, 97–107, https://doi.org/10.1111/nph.14331, 2017.
Saiz, E., Sgouridis, F., Drijfhout, F. P., and Ullah, S.: Biological nitrogen fixation in peatlands: Comparison between acetylene reduction assay and 15N2 assimilation methods, Soil Biol. Biochem., 131, 157–165, https://doi.org/10.1016/j.soilbio.2019.01.011, 2019.
Saiz, E., Sgouridis, F., Drijfhout, F. P., Peichl, M., Nilsson, M. B., and Ullah, S.: Chronic atmospheric reactive nitrogen deposition suppresses biological nitrogen fixation in peatlands, Environ. Sci. Technol., 55, 1310–1318, https://doi.org/10.1021/acs.est.0c04882, 2021.
Sanda, M. and Cislerova, M.: Transforming hydrographs in the hillslope subsurface, J. Hydrol. Hydromech., 57, 264–275, https://doi.org/10.2478/v10098-009-0023-z, 2009.
Schwintzer, C. R.: Nonsymbiotic and symbiotic nitrogen fixation in a weakly minerotrophic peatland, Am. J. Botan., 70, 1071–1078, https://doi.org/10.1002/j.1537-2197.1983.tb07908.x, 1983.
Sgouridis, F., Yates, C. A., Lloyd, C. E., Saiz, E., Schillereff, D. N., Tomlinson, S., Williamson, J., and Ullah, S.: Chronic atmospheric reactive N deposition has breached the N sink capacity of a northern ombrotrophic peatbog increasing the gaseous and fluvial N losses, Sci. Total Environ., 787, 147552, https://doi.org/10.1016/j.scitotenv.2021.147552, 2021.
Song, L., Lu, H. Z., Xu, X. L., Li, S., Shi, X. M., Chen, X., Wu, Y., Huang, J.-B., Chen, Q., Wu, C.-S., and Liu, W. Y.: Organic nitrogen uptake is a significant contributor to nitrogen economy of subtropical epiphytic bryophytes, Sci. Rep., 6, 1–9, https://doi.org/10.1038/srep30408, 2016.
Soper, F. M., Simon, C., and Jauss, V.: Measuring nitrogen fixation by the acetylene reduction assay (ARA): Is 3 the magic ratio?, Biogeochemistry, 152, 345–351, https://doi.org/10.1007/s10533-021-00761-3, 2021.
Stuart, J. E., Holland-Moritz, H., Lewis, L. R., Jean, M., Miller, S. N., McDaniel, S. F., Fierer, N., Ponciano, J. M., and Mack, M. C.: Host identity as a driver of moss-associated N2 fixation rates in Alaska, Ecosystems, 24, 530–547, https://doi.org/10.1007/s10021-020-00534-3, 2021.
Tacheci, P.: Hydrology in a headwater catchment and prediction of the effect of deforestation, Czech Technical University in Prague, Ph.D. Thesis, 144 pp., 2002 (in Czech).
Urban, N. R. and Eisenreich, S. J.: Nitrogen cycling in a forested Minnesota bog, Can. J. Botan., 66, 435–449, https://doi.org/10.1139/b88-069, 1988.
van den Elzen, E., Kox, M. A., Harpenslager, S. F., Hensgens, G., Fritz, C., Jetten, M. S., Ettwig, K. F., and Lamers, L. P.: Symbiosis revisited: Phosphorus and acid buffering stimulate N2 fixation but not Sphagnum growth, Biogeosciences, 14, 1111–1122, https://doi.org/10.5194/bg-14-1111-2017, 2017.
van den Elzen, E., van den Berg, L. J., van der Weijden, B., Fritz, C., Sheppard, L. J., and Lamers, L. P.: Effects of airborne ammonium and nitrate pollution strongly differ in peat bogs, but symbiotic nitrogen fixation remains unaffected, Sci. Total Environ., 610, 732–740, https://doi.org/10.1016/j.scitotenv.2017.08.102, 2018.
van den Elzen, E., Bengtsson, F., Fritz, C., Rydin, H., and Lamers, L. P.: Variation in symbiotic N2 fixation rates among Sphagnum mosses, PLoS ONE, 15, e0228383, https://doi.org/10.1371/journal.pone.0228383, 2020.
Vile, M. A., Bridgham, S. D., Wieder, R. K., and Novak, M.: Atmospheric sulfur deposition alters pathways of gaseous carbon production in peatlands, Global Biogeochem. Cy., 17, 1058, https://doi.org/10.1029/2002GB001966, 2003.
Vile, M. A., Wieder, K. R., Zivkovic, T., Scott, K. D., Vitt, D. H., Hartsock, J. A., Iosue, C. L., Quinn, J. C., Petix, M., Fillingim, H. M., Popma, J. M. A., Dynarski, K. A., Jackman, T. R., Albright, C. M., and Wykoff, D. D.: N2-fixation by methanotrophs sustains carbon and nitrogen accumulation in pristine peatlands, Biogeochemistry, 121, 317–328, https://doi.org/10.1007/s10533-014-0019-6, 2014.
Violaki, K., Zarbas, P., and Mihalopoulos, N.: Long-term measurements of dissolved organic nitrogen (DON) in atmospheric deposition in the Eastern Mediterranean: Fluxes, origin and biogeochemical implications, Mar. Chem., 120, 179–186, https://doi.org/10.1016/j.marchem.2009.08.004, 2010.
Vitousek, P. M. and Howarth, R. W.: Nitrogen limitation on land and in the sea: how can it occur?, Biogeochemistry, 13, 87–115, https://doi.org/10.1007/BF00002772, 1991.
Vitt, D. H., Wieder, K., Halsey, L. A., and Turetsky, M.: Response of Sphagnum fuscum to nitrogen deposition: a case study of ombrogenous peatlands in Alberta, Canada, Bryologist, 106, 235–245, https://doi.org/10.1639/0007-2745(2003)106[0235:ROSFTN]2.0.CO;2, 2003.
Vitvar, T., Jankovec, J., and Sanda, M.: Revealing subsurface processes in the Uhlirska catchment through combined modelling of unsaturated and saturated flow, Hydrol. Process., 36, e14516, https://doi.org/10.1002/hyp.14516, 2022.
Walbridge M. R. and Navaratnam, J. A.: Phosphorous in boreal peatlands, in: Boreal Peatland Ecosystems, edited by: Wieder, R. K. and Vitt, D. H., Springer Berlin Heidelberg New York, 231–258, ISBN-13 978-3-540-31912-2, 2006.
Wang, Y., Lett, S., and Rousk, K.: Too much of a good thing? Inorganic nitrogen (N) inhibits moss-associated N2 fixation but organic N can promote it, Biogeochemistry, 159, 179–191, https://doi.org/10.1007/s10533-022-00920-0, 2022.
Warren, M. J., Lin, X., Gaby, J. C., Kretz, C. B., Kolton, M., Morton, P. L., Pett-Ridge, J., Weston, D. J., Schadt, C. W., Kostka, J. E., and Glass, J. B.: Molybdenum-based diazotrophy in a Sphagnum peatland in northern Minnesota, Appl. Environ. Microbiol., 83, e01174-17, https://doi.org/10.1128/AEM.01174-17, 2017.
Wieder, R. K.: Element stoichiometry and nutrient limitation in bog plant and lichen species, Biogeochemistry, 160, 355–379, https://doi.org/10.1007/s10533-022-00968-y, 2022.
Wieder, R. K. and Vitt, D. H.: Boreal Peatland Ecosystems, Springer Berlin Heidelberg New York, ISBN-13 978-3-540-31912-2, 2006.
Wieder, R. K., Vitt, D. H., Vile, M. A., Graham, J. A., Hartsock, J. A., Fillingim, H., House, M., Quinn, J. C., Scott, K. D., Petix, M., and McMillen, K. J.: Experimental nitrogen addition alters structure and function of a boreal bog: Critical load and thresholds revealed, Ecol. Monogr., 89, e01371, https://doi.org/10.1002/ecm.1371, 2019.
Wieder, R. K., Vitt, D. H., Vile, M. A., Graham, J. A., Hartsock, J. A., Popma, J. M., Fillingim, H., House, M., Quinn, J. C., Scott, K. D., Petix, M., and McMillen, K. J.: Experimental nitrogen addition alters structure and function of a boreal poor fen: Implications for critical loads, Sci. Total Environ., 733, 138619, https://doi.org/10.1016/j.scitotenv.2020.138619, 2020.
Yin, T., Feng, M., Qiu, C., and Peng, S.: Biological nitrogen fixation and nitrogen accumulation in peatlands, Front. Earth Sci., 10, 670867, https://doi.org/10.3389/feart.2022.670867, 2022.
Zeileis, A.: Econometric computing with HC and HAC covariance matrix estimators, J. Stat. Softw., 11, 1–17, https://doi.org/10.18637/jss.v011.i10, 2004.
Zhang, X., Ward, B. B., and Sigman, D. M.: Global nitrogen cycle: Critical enzymes, organisms, and processes for nitrogen budgets and dynamics, Chem. Rev., 120, 5308–5351, https://doi.org/10.1021/acs.chemrev.9b00613, 2020.
Zielke, M., Solheim, B., Spjelkavik, S., and Olsen, R. A.: Nitrogen fixation in the high arctic: role of vegetation and environmental conditions, Arct. Antarct. Alp. Res., 37, 372–378, 2005.
Zivkovic, T., Disney, K., and Moore, T. R.: Variations in nitrogen, phosphorus, and δ15N in Sphagnum mosses along a climatic and atmospheric deposition gradient in eastern Canada, Botany, 95, 829–839, https://doi.org/10.1139/cjb-2016-0314, 2017.
Zivkovic, T., Helbig, M., and Moore, T. R.: Seasonal and spatial variability of biological N2 fixation in a cool temperate bog, J. Geophys. Res.-Biogeo., 127, e2021JG006481, https://doi.org/10.1029/2021JG006481, 2022.