the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Organic-matter-mediated leaching of alkalinity in limed acid soils is affected by dissolved organic carbon adsorption and soil structure
Hannah Van Ryckel
Lynn Van Aelst
Toon van Dael
Erik Smolders
Subsurface soil acidity severely limits crop growth and is challenging to adjust by surface liming. There have been several proposals for subsurface liming using the combination of lime and an organic amendment, as organic anions may migrate deeper in acid subsoil than carbonates. This study aimed to identify mechanisms of subsurface liming, postulating that it is hindered by dissolved organic carbon (DOC) adsorption but enhanced in structured compared to sieved soils due to preferential flow in macropores. Column leaching experiments were set up using three sieved acid soils with contrasting properties, one of which was additionally sampled as undisturbed soil cores. The upper layer of each soil was treated with lime, compost, or a combination of both, in addition to an untreated control, and columns were leached with artificial rainwater. Deeper subsurface liming in the lime + compost treatment than in the lime treatment was detected in only one of the three soils. The effect of compost on the migration of alkalinity was explained by differences in DOC sorption among soils, with the lowest sorption leading to deepest subsurface liming. Imaging of in situ pH using a planar optode showed evidence of preferential alkalinity flow in the structured soil; however, destructive sampling of bulk soil layers did not confirm this. We conclude that combining lime with an organic amendment can effectively ameliorate subsoil acidity but this requires weakly DOC-adsorbing subsoils. The role of soil structure in this process needs to be corroborated with plant responses to identify benefits of liming the macropores.
- Article
(1504 KB) - Full-text XML
-
Supplement
(1464 KB) - BibTeX
- EndNote
About half of the world's potential arable land consists of acid soils (pH≤5.5), making soil acidity one of the most important agricultural constraints worldwide (George et al., 2012; Kochian et al., 2004; von Uexküll and Mutert, 1995). Toxic levels of aluminum (Al) and manganese (Mn) and deficiencies in phosphorus (P), calcium (Ca), and magnesium (Mg) severely affect crop yield in acid soils. Restricted root growth, particularly caused by Al, leads to even lower nutrient uptake and increased water stress (Marschner, 1991; Tang et al., 2013). Topsoil acidity is typically amended by applying lime or dolomite (CaCO3 or CaMg(CO3)2). However, surface application of lime is often inefficient in alleviating subsoil acidity (below 0.1 m) due to the slow downward movement of lime in soil (Conyers and Scott, 1989; Sumner et al., 1986; Tang et al., 2013). The factors explaining the low mobility of lime are its low solubility, the fast consumption of OH− or produced during the liming reaction, and the lack of an accompanying anion for the downward transport of Ca2+ (Liu and Hue, 2001). For example, Azam and Gazey (2020) found that the subsurface soil pH increase remained limited to 0.049 pH units per year to a maximum depth of 0.20 m. This was only achieved after repeated surface applications of unrealistically high doses of lime (up to a total of 8.5 t ha−1) over 10–24 years. Similarly, Li et al. (2019) showed in an 18-year field trial that pH increase in the soil profile remained confined to the top 0.3 m depth when pH was continuously maintained above 5.5 in the top 0.1 m. Consequently, the adverse effects of soil acidity often persist in the root zone, which becomes particularly important when moisture is depleted in the topsoil at the end of the growing season, and plants need to rely on water and nutrients from the more acid subsoil (Tang et al., 2003, 2013). Therefore, many studies have searched for ways to alleviate subsoil acidity.
Limited evidence suggests that combining lime with an organic amendment (OA) might enhance alkalinity movement down the soil profile (Butterly et al., 2021; Lauricella et al., 2021; Liu and Hue, 2001; Miyazawa et al., 2002; Wright et al., 1985). Even on its own, an OA can increase soil pH, depending on the type of residue, its rate of application, and the buffer capacity of the soil (Haynes and Mokolobate, 2001). The main mechanisms for this acid-neutralizing effect are (i) the proton uptake of the organic anions of humic substances that act as weak bases, (ii) decarboxylation of organic acids during residue decomposition, and (iii) ammonification of residue nitrogen (N) (Haynes and Mokolobate, 2001; Wong and Swift, 2003; Yan et al., 1996). Conjugated bases of organic acids derived from the added residues (further referred to as organic anions) can leach down the soil profile and increase soil pH in deeper layers due to continued decarboxylation and ammonification (Butterly et al., 2021; Tang et al., 2013). However, their effect on soil pH is variable and prone to re-acidification, as the initial pH increase is often followed by a decrease when nitrification occurs (Yan et al., 1996). When combining lime with an OA, the temporary increase in pH in deeper soil layers, caused by the OA, reduces the pH gradient between the limed layer and that below. This can be sufficient for lime-derived alkalinity to leach out from the amended zone and bring about a more long-lasting increase in pH in the subsoil (Butterly et al., 2021). Additionally, the organic anions can function as the accompanying anion for the transport of lime-derived Ca2+. This calcium can replace exchangeable H+ and Al3+ in the subsoil, thereby further increasing soil pH and decreasing Al toxicity (Haynes and Judge, 2008; Hue and Licudine, 1999; Smith et al., 1995; van der Watt et al., 1991). For example, Lauricella et al. (2021) found in a column leaching experiment that soil pH in columns amended with the combination of lime and vegetable garden compost increased by 0.14 units in the first 2 cm below the amended zone and 0.08 units in the 3 cm below that compared to the lime-only control. However, very little is known about the factors influencing organic-matter-mediated alkalinity leaching in soil. The success of this process likely depends on the soil's affinity for retaining the organic matter. Binding of DOC occurs on free binding sites of Fe and Al oxyhydroxides (Kindler et al., 2011). The available binding sites can be inferred from the amorphous Fe + Al content in the soil, corrected for anions that already occupy these sites. These anions are mainly phosphate and organic anions (RO−) (Verbeeck et al., 2017). In spite of this, the specific influence of the ratio on organic-matter-mediated lime leaching has never been tested. This gap is particularly relevant as subsoil acidity is an important issue in weathered soils with low ratios (Kögel-Knabner and Amelung, 2014).
This study was set up to identify the mechanisms of subsurface liming imposed by applying the combination of lime and organic amendments at the soil surface. Our first hypothesis is that alkalinity leaching is negatively influenced by DOC adsorption in the subsoil (i.e., under the treated soil), so in soils with large DOC solid–liquid distribution coefficients (KD values). Additionally, most research on subsurface liming has focused on leaching in sieved soils, despite evidence that preferential flow through macropores in intact soils can greatly increase chemical leaching (e.g., pesticides, fertilizers, trace metals) compared to matrix flow in sieved soils (Jacobsen et al., 1997; Jarvis, 2007; de Jonge et al., 2004; Lægdsmand et al., 1999; Paradelo et al., 2013; White, 1985). Our second hypothesis is that preferential flow in macropores enhances leaching of alkalinity, meaning that non-dissolved lime particles and lime-bound organic anions could enhance subsoil pH more in intact soils than in sieved ones, especially in short-term lab experiments. Two consecutive column leaching experiments were set up to test these hypotheses. In both experiments, soils were packed in columns, with the topsoil layer treated for each soil with CaCO3, an organic amendment, or a combination of both, in addition to an untreated control, after which the columns were leached with artificial rainwater. Two acid soils with contrasting KD values of the DOC (a Podzol and a Ferralsol) were used in the first experiment. In the second experiment, a third acid soil (a Retisol) was used, with half of the columns sampled as intact soil cores and half packed with the same soil after sieving. These three soils were selected as representative examples of soils exhibiting low, average, and high DOC sorption, with the intention of creating a gradient in the success of organic-matter-mediated leaching.
2.1 Soil sampling and preparation
Three acid soils were sampled at different locations (Table 1). The first one was sampled from a Podzol in a clear-cut area after 40 years of Norway spruce (Picea abies L.) on former agricultural land in Riel, the Netherlands. The second one was sampled from a Ferralsol in uncultivated land in Da Loan, Vietnam. The third one was sampled from a Retisol in a forest in Bertem, Belgium. In Riel and Da Loan, bulk soil was sampled from the top 20 cm. In Bertem, sampling of bulk soil and an additional sampling of six undisturbed soil columns were performed below the organic layer (forest floor). The undisturbed columns were 14 cm in soil height and were carefully transported to the lab to avoid soil structure disturbance. All bulk soil was air-dried and sieved to 2 mm.
Table 1Selected characteristics of the soils used in the three-column leaching experiments including the DOC solid–liquid distribution coefficient KD.
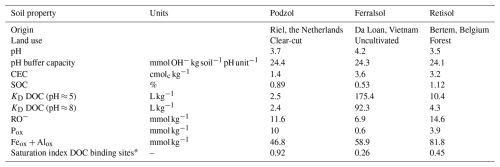
* Eq. (1).
Soil pH was determined in 0.01 M CaCl2 (1:5 solid–liquid ratio). Soil buffer capacity was defined as the slope of the curve (Fig. S1 in the Supplement) of the amount of base added to the soil () as a function of soil pH (0.001 M CaCl2, 1:5) when the soil was limed to different degrees with Ca(OH)2 until a pH of 5.5. The cation exchange capacity (CEC) was determined using the cobalt hexamine method (Protocol ISO 23470, 2007). Total soil organic carbon (%SOC) was determined on oven-dried samples at 105 °C with an elemental analyzer (Carlo Erba EA1108) in tin capsules. The concentrations of amorphous Al and Fe oxyhydroxides (and the P associated with them) were determined with oxalate extraction on soil samples dried at 45 °C according to Schwertmann (1991), followed by measurement with ICP-OES (ICP-OES Thermo Scientific iCAP 7000 series). All these soil analyses included internal soil reference materials and analytical replicates to ensure accuracy and precision. All results are reported on a dry weight (105 °C) basis. Additional soil properties with a description of corresponding analysis methods are given in Table S1 in the Supplement.
A combined index was calculated from the soil analyses to rank the soils in terms of the strength of net DOC sorption. The adsorption of DOC in soils is likely the result of sorption of binding sites of dissolved humic substances to free binding sites on Fe and Al oxyhydroxides. These binding sites are commonly determined as half of the sum of molar-oxalate-extractable Fe and Al (Feox, Alox, mmol kg−1) corrected for oxalate extractable P on these sites (Pox, mmol kg−1) (Renneson et al., 2015). Part of the soil organic carbon also occupies these sites and anion sorption studies on soil have suggested that the competing reactive organic anion (RO−, mmol kg−1) is 1.3 total soil organic carbon present in the soil (Verbeeck et al., 2017). Hence, the saturation index of the DOC binding sites can be calculated from the oxalate extracts of the soil and from the SOC content as
Adsorption isotherms of DOC were constructed for each soil at three different soil pH levels: native pH, pH≈5, and pH≈8. The OA used as a DOC source in the adsorption test was a green compost originating from ILVO in Ghent, Belgium. The compost had a pH of 8.8 (0.01 M CaCl2, 1:5 solid–liquid ratio). The DOC concentration was determined by extracting the compost with 0.001 M CaCL2 at a solid–liquid ratio of 1:10. Samples were shaken for 2 h, centrifuged for 15 min at 1400 RCF, and filtered through 1.2 µm Chromafil filters. The DOC concentration in the extract was 781 mg DOC L−1 and was measured using the combustion catalytic oxidation method (Shimadzu TOC-L CPH). The degree of aromaticity of the samples was 30 %, determined using the specific UV absorbance (SUVA), as detailed in Amery et al. (2010). The OA was selected from a range of OAs (Table S2) based on its high DOC concentration, to maximize the leaching of organic anions, and low aromaticity, to minimize sorption to Fe and Al oxides in the soil. For the DOC adsorption tests, soils were first mixed with Ca(OH)2 at the correct doses. For each soil and Ca(OH)2 dose, aliquots of 3 g were mixed with 30 mL of a 0.001 M CaCl2 solution with increasing compost-derived DOC (extracted from the compost in advance as described above and diluted with 0.001 M CaCl2 to varying initial DOC concentrations (0–781 mg DOC L−1)). Soil suspensions were shaken for 16 h, centrifuged at 1400 RCF for 10 min, and filtered through a 1.2 µm Chromafil filter. The DOC concentration in the filtrate was determined with a TOC analyzer (Shimadzu TOC-L CPH). A modified Langmuir equation was used to describe the sorption isotherms, according to Siemens et al. (2004):
where s′ is the desorbed or adsorbed DOC (mg kg−1), is a parameter for the maximum sorbed DOC (mg kg−1), k′ is an affinity parameter (L mg−1), c is the concentration of DOC in solution (mg L−1), and C is a parameter for the desorbable amount of soil DOC (mg kg−1). In the Ferralsol, the DOC concentrations were always lower after than before the reaction, i.e., there was a net DOC adsorption, and the parameter C was not significantly different from zero. Conversely, in the Podzol and Retisol, DOC concentrations were always higher after reaction with the soils than before, i.e., net DOC desorption from soil occurred, even at high added DOC concentrations. This was accounted for by a parameter C significantly different from zero in these soils. The fitted Langmuir adsorption isotherms are shown in Fig. S2. The linear parts of these curves were summarized with the initial slope, i.e., the solid–liquid distribution coefficient KD (L kg−1), and the linear parts were used by considering all the points between 0–25 mg DOC L−1 (Ferralsol) and 0–150 mg DOC L−1 (Podzol and Retisol). A calculation of the initial DOC concentration in the soil solution upon adding the OA to the soil in the column experiment described below showed initial DOC concentrations of 0.6 mg L−1 (Ferralsol), 33.9 mg L−1 (Podzol), and 9.7 mg L−1 (Retisol); i.e., these soil + compost mixtures were within that linear part of the curves.
2.2 Setup of the column experiments
Two consecutive leaching experiments were performed in a column setup previously described in detail in Bergen et al. (2023). This setup maintains unsaturated conditions by placing the columns on suction plates and applying a mild vacuum at the outlet. Although this approach limits the number of replicates and thus reduces statistical power, it offers a more robust alternative to free drainage systems, which lead to water saturation near the outlet and can therefore cause artifactual changes in soil pH (Lewis and Sjöstrom, 2010). In short, plexiglass cylinders of 6 cm diameter were filled with soil until a height of 16 cm. The upper 2 cm of each soil column was treated either with CaCO3 at a dose of 5 g kg−1, an OA at a dose of 10 g dry matter kg−1, or a combination of both, in addition to an untreated control. The OA was the green compost from ILVO, Ghent, described in the previous section. The doses of CaCO3 and OA correspond to a field application rate of 7 and 14 t ha−1 when assuming a bulk density of 1.4 t m−3. In Experiment 1, one replicate was included for the control and OA treatments, while two replicates were included for the lime and lime + OA treatments, resulting in 12 columns. In Experiment 2, the same design was made and half of the columns (n=6) were sieved while the other half were intact (n=6), with a 2 cm (un)treated layer of the sieved soil added on top. The soil densities after filling of the columns were 1.35 g cm−3 (Podzol), 1.15 g cm−3 (Ferralsol), 1.05 g cm−3 (Retisol, sieved), and 1.29 g cm−3 (Retisol, intact). The soil columns were wetted to field capacity and placed on ceramic plates pre-wetted with ultrapure Milli-Q water. The columns and plates were then placed in PVC housing with rubber rings for sealing. The bottom of each housing was attached to an Erlenmeyer flask to collect the percolate. The Erlenmeyer flasks were connected to a vacuum pump, which maintained the pressure at 900 mbar to achieve unsaturated flow conditions. Each column was irrigated with artificial rainwater composed of 1 mM CaCl2, 0.003 mM KOH, 0.02 mM NaOH, and 0.02 mM H2SO4 (pH of 5.26) from a separate container through a peristaltic pump (Watson–Marlow 205 U). The average simulated rainfall intensity was 2 mm d−1. In Experiment 1, columns were leached for 3 weeks with a total leaching of 0.61 pore volumes for the Podzol and 0.53 pore volumes for the Ferralsol. In Experiment 2, the aim was to reach a total leaching of 2 pore volumes to enhance alkalinity leaching. Therefore, columns were leached for 11 weeks with a total leaching of 1.8 pore volumes for the sieved Retisol and 2.13 pore volumes for the intact Retisol.
2.3 Column dismantling and soil analyses
At the end of each experiment, the columns were dismantled. In Experiment 1, the soil columns were sliced at 1 cm intervals until a depth of 16 cm. Soil pH at each depth was measured in 0.01 M CaCl2 at a 1:5 solid–liquid ratio after 2 h of shaking. The DOC concentration at each depth was determined by performing a 1:1 solid–liquid extraction with 0.001 M CaCl2. The DOC concentration of the extracts was measured via the combustion catalytic oxidation method (Shimadzu TOC-L CPH) after samples were shaken for 30 min, centrifuged for 15 min at 1400 RCF, and filtered over 0.45 µm Chromafil filters. In Experiment 2, the soil column was removed from the plexiglass cylinder and carefully sliced lengthwise into two equal parts with a galvanized iron wire. Half of the column was used to determine the pH and DOC concentration as a function of the depth as described for Experiment 1. The other half was used to image the in situ soil pH in two dimensions using a planar optode (PO).
2.4 Planar optode imaging
Imaging of the in situ soil pH along the depth profile was accomplished using a PO system (“VisiSens TD”, PreSens GmbH, Regensburg, Germany) in Experiment 2. A planar optode is an optical device that uses sensor foils containing an analyte-sensitive dye immobilized in an analyte-permeable matrix brought into contact with the sample. When excited by a light source, the dye emits a fluorescence signal that changes dynamically with varying analyte concentrations. A digital camera captures the signal, and the software translates it into a color image of the analyte distribution (Kreuzeder et al., 2018; Li et al., 2019; Santner et al., 2015; Tschiersch et al., 2011). In this study, pH-sensitive foils (7 cm×2.5 cm) were fixed to a glass plate and applied to the cut-open half of the soil column. The foils were left to equilibrate with the soil solution for 24 h before imaging. The PO was calibrated with 12 citrate buffers at an ionic strength (IS) of 25 mM and a pH ranging from 3.06–5.36. This IS was chosen to mimic the IS of the soil solution in the samples.
2.5 Statistical analysis
Statistical differences in pH values among soil slices (=depth) within the same column were determined by ANOVA followed by a Dunnett's test ( depth) at 0.05 level of significance. Treatment effects on the pH profiles were analyzed with a functional approach, i.e., nonlinear regression. First, all pH values of soil treatments were corrected to that of the untreated control. This yielded ΔpH(depth) data, i.e., the difference in pH between a treated soil and the untreated control at the corresponding depth. The ΔpH(depth) exhibited a sigmoidal decreasing trend towards depth under the treated layer, and this was fitted with the following four-parameter model:
where ΔpHmax (–) is the maximum value of ΔpH, ΔpHback (–) is the background ΔpH, slope (cm−1) represents the steepness of the curve, and b (cm) is the inflection point of the curve.
Equation (4) shows that ΔpHmax is the asymptote at the soil surface (depth=0 cm):
when .
The ΔpH–depth profiles were fitted for every treatment within each soil with the nonlinear fitting option in the JMP software (JMP pro 17, SAS Institute Inc.). For the OA + lime and lime treatments, data for all four columns were fitted in one set with an assumed difference in parameter values for each of the four parameters. The statistical differences in parameter values were tested to identify treatment effects on the extent and depth of penetration of the alkalinity. The depth profiles of the ΔDOC concentrations were fitted by replacing ΔpH with ΔDOC in Eq. (3) (see the Supplement).
3.1 Soil properties
Selected soil properties are given in Table 1. All three soils were acid (pH 3.4–4.2). The distribution coefficients of DOC at a common pH=5 ranked Ferralsol > Retisol > Podzol and varied over an order of magnitude. This ranking also follows the ranking of the saturation index for DOC binding sites (a high value indicates low sorption). Other soil characteristics can be found in Table S1.
3.2 Soil pH
The pH of the treated topsoil (0–2 cm) was increased with 2.3–3.5 units above the control by lime or lime + OA addition in all soils (Fig. 1 and Tables S3 and S4). Alkalinity movement down the soil profile was observed in the first layer below the treated layer (2–3 cm). Red asterisks in Fig. 1 mark ΔpH values that are significantly higher than the ΔpH value of the 5–6 cm layer of the same soil column, indicating an increase in pH compared to the original pH in the unamended part of the soil column. In the Podzol, soil pH in the 2–3 cm layer increased, on average, 0.6 units in the limed treatment and 1.5 units in the lime + OA treatment compared to the original pH in the respective columns. The inflection point of the sigmoidal curve (i.e., parameter b in Eq. 3) was significantly larger (=deeper) in the lime + OA treatment than in the limed treatment in this soil, indicating larger alkalinity leaching in the former (details not shown). In the Ferralsol, no such increase in pH compared to the original pH was observed below the treated layer. In the sieved and intact Retisol, soil pH in the 2–3 cm layer of the lime + OA treatment was significantly higher than in deeper layers, whereas that increase was not statistically significant in the lime-only treatment. The lime + OA treatment increased pH by 1.3 units in the sieved soil and 0.9 units in the intact soil. The functional analyses of the ΔpH did not show a significantly larger penetration of alkalinity (p>0.05) of in the lime + OA compared to the lime-only in the Retisol.
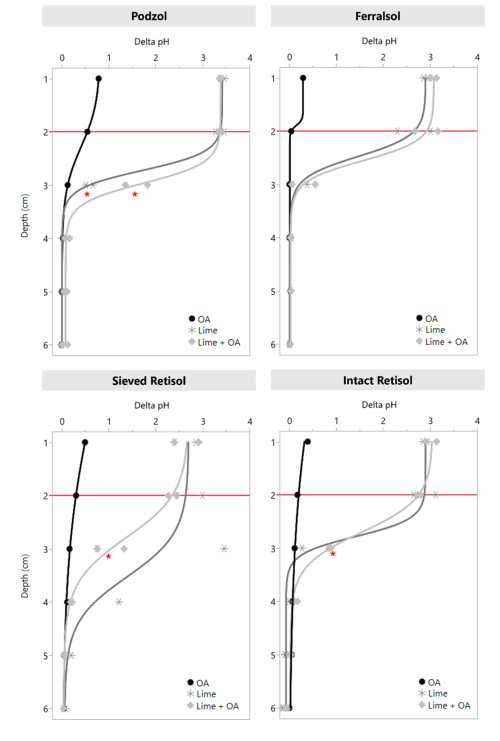
Figure 1Data points and sigmoidal fits (Eq. 3) of the depth profiles of ΔpH (difference between pH in a specific soil layer and the pH of the control treatment in the corresponding soil layer) values in soil slices after dismantling of the columns. The red horizontal lines represent the border of the treated layer. Red asterisks represent significantly higher ΔpH values (Dunnett, p<0.05) than the ΔpH value of the 5–6 cm soil layer of the same column.
The in situ pH in the columns measured by the planar optode is shown in Fig. 2. Only three columns were selected for illustrative purposes: the control treatment, a limed treatment, and a lime + OA treatment of the intact Retisol. The movement of the alkalinity front down the soil profile is visible, with deeper leaching of the alkalinity in the lime + OA treatment than in the lime-only treatment. The absolute values of the soil pH are solely indicative since incomplete contact between the sample and sensor foil may occur in unsaturated samples. The upper 2 cm layer in the intact columns consists of sieved soil with a slightly higher pH than that of the intact soil below the treated layer due to drying and rewetting, explaining the yellow-red color of the upper 2 cm layer in the control treatment.
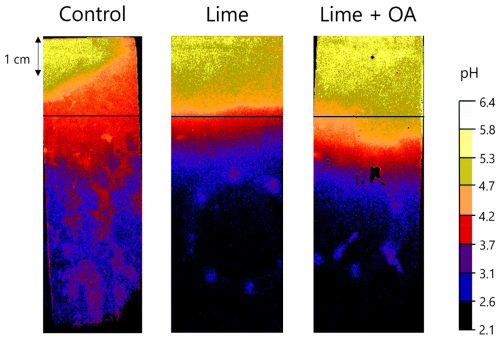
Figure 2Planar optode image of in situ soil pH for control, lime, and lime + OA treatments in the intact Retisol. The top of the image coincides with the soil surface. The horizontal black lines indicate the border of the treated layer. Note that the treated layer is a 2 cm layer of sieved soil (higher pH, Table 1) imposed on the intact column, hence the increased soil pH in the upper 2 cm of the control treatment.
3.3 DOC concentrations
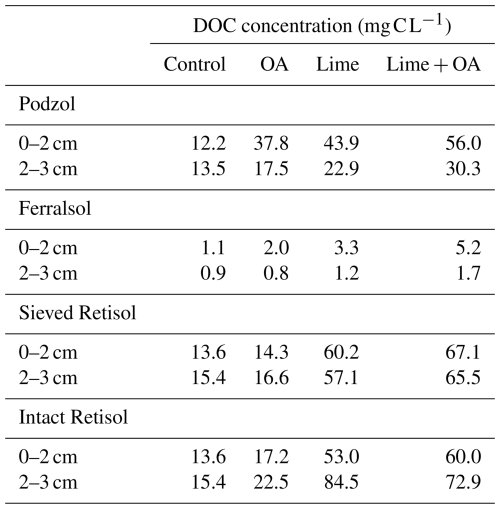
Clear trends in DOC concentrations are shown in Table 2 and Fig. S3. Generally, DOC concentrations were raised by the soil amendments in the following order: lime + OA > lime ≫ OA. The factor increase in DOC among the treatments was similar across all soils. However, absolute DOC concentrations in the Ferralsol remained markedly lower than in the other soils.
4.1 Effect of DOC adsorption on alkalinity leaching
The results of this study indicate that combining lime with an OA leads to enhanced alkalinity leaching to the subsoil compared to a lime-only treatment (Fig. 1 and Tables S3 and S4). In the Podzol, combining lime with an OA increased the pH in the 2–3 cm layer more than twice as much as applying lime alone. Moreover, in the Retisol, statistically significant pH increases below the treated layer were only observed when lime was combined with an OA. However, no alkalinity leaching was observed in the Ferralsol. This soil type is characterized by a high Fe and Al (hydr)oxide content due to intense soil weathering (Kögel-Knabner and Amelung, 2014). These Fe and Al oxides are considered to be the most important adsorbents for DOC in soils. Therefore, the oxalate-extractable Fe and Al content in soil is a good indicator of the DOC adsorption capacity, when corrected for the already adsorbed species (mainly Pox and RO−) (Kaiser et al., 1996; Kindler et al., 2011; Moore et al., 1992). On top of that, weathered soils typically contain clay minerals like kaolinite, which are also important adsorbents for DOC in soils (Jardine et al., 1989; Kalbitz et al., 2000). Indeed, the KD value of the DOC adsorption isotherms (Table 1 and Fig. S2) was considerably larger in the Ferralsol than in the two other soils. Table 2 shows that the DOC concentrations measured in this soil ranged from to about 5 mg C L−1, while typical DOC concentrations in soil solution range from 1–50 mg C L−1 (Herbert and Bertsch, 1995). The low concentration of organic anions in the solution probably prevented the facilitated transport of lime-derived alkalinity, confirming our first hypothesis. Our results imply that DOC-mediated alkalinity leaching is unlikely to occur in weathered soils, typically located in the humid tropics (Werts, 2023). This is an unfortunate outcome, as most of the potentially arable acid soils are located in the humid tropics, and resource-limited farmers in these areas could particularly benefit from cost-effective solutions to remediate (sub)soil acidity (von Uexküll and Mutert, 1995).
Table 2DOC concentrations in soil extracts from the treated layer (0–2 cm) and the layer below (2–3 cm) after dismantling of the columns.
The pH effects in the subsoil are logically rather small due to the limited duration of column experiment, i.e., on 0.5–2.1 pore volumes. Two short calculation examples are added to estimate long-term impacts of the organic amendments for subsurface liming. For any adsorbing compound, in this case DOC, a retardation factor R represents the time required to travel through the soil relative to that of water and is defined as with ρ the density of the absorbent (kg L−1), KD the solid–liquid distribution coefficient (L kg−1) and θ the volumetric moisture content (–). Since water travels about 2.3 m yr−1 (assuming a net drainage excess in tropical soils of 0.7 m, and a volumetric moisture content of 0.3), it follows that DOC leaches 2.3 m/R annually. With the given DOC KD value at pH 5 (Table 1), a soil bulk density of 1.3 kg L−1, and a volumetric moisture content of 0.3, the DOC leaching depth is only 3.1 mm yr−1 for the Ferralsol. The adsorption of DOC can be lowered by increasing soil pH through liming due to the decreasing positive charge on the Fe and Al (hydr)oxides in the soil at increasing pH (Tipping, 1981). Indeed, when recalculating for pH 8, a DOC leaching depth of 5.8 mm yr−1 is found. Although the yearly leaching depth almost doubled by increasing the pH from 5–8, it would still take about 17 years before the DOC has leached to the subsoil (>0.1 m) in the case of surface application.
The second calculation is based on the total alkalinity generated by the DOC leaching to deeper layers in the longer term. First, the quantity of negatively charged ligands originating from the added DOC in the topsoil layer that leaches to the subsoil in the Ferralsol is calculated using Visual MINTEQ (Gustafsson, 2000). The IS was fixed at 0.001 M and Ca2+ was added at a total concentration of 0.001 M. The DOC was included using the Nica–Donnan model, at 5 mg C L−1, in line with the measured DOC concentration in the topsoil layer of the lime + OA treatment (Table 2). The model was run at pH 7 and at pH 4.2, corresponding to the pH of the topsoil and subsoil layer in the lime + OA treatment (Table S3). The concentration of Ca2+ bound to DOC was in the topsoil and in the subsoil. The difference between these two values is the concentration alkalinity released upon this pH change, or Ca2+. The DOC that was bound to this Ca can bind two protons for each Ca2+ ion released, meaning that the quantity of negatively charged ligands able to bind protons in the subsoil is . A subsoil layer of 10 cm in depth, 1 m2 in surface area, and with a bulk density of 1.3 kg L−1 was considered. With a net drainage excess of 700 L yr−1 through this unit area, the negative charge leaching to the subsoil is 10 mmol yr−1. At a total amount of 130 kg soil in the subsoil layer under consideration, this corresponds to 0.08 soil that can contribute to neutralizing the protons in the subsoil. The pH increase in the subsoil layer for the Ferralsol with a buffer capacity of 24.3 soil pH unit−1 (Table 1) is then equal to 0.003 pH units yr−1, i.e., vanishingly small. Considering these values, it would take over 300 years to increase the pH in the subsoil with 1 pH unit in the Ferralsol. In reality, increasing soil pH in the strongly sorbing Ferralsol would be even more difficult due to fixation of DOC on the soil matrix (see first calculation example). These simplistic calculations show that DOC-mediated alkalinity leaching is unlikely to happen at realistic timescales in soils such as the Vietnamese Ferralsol with high concentrations of DOC reactive binding sites.
4.2 Effect of soil structure on alkalinity leaching
The pH maps with the planar optodes revealed that alkalinity migrates deeper with OA in structured soils. However, bulk soil measurements did not confirm increased alkalinity leaching in structured soils compared to that in sieved soils. This is in contrast to our hypothesis that enhanced alkalinity leaching would take place in the structured Retisol, driven by (i) non-equilibrium transport of dissolved organic anions complexed with lime-derived Ca2+ and (ii) preferential particle transport of non-dissolved lime particles and mobile colloids containing organic matter, possibly bound to Ca2+ through ligand exchange on acid functional groups. Previous studies did show enhanced chemical leaching in structured soils, attributed to macropores (pores larger than ∼0.3 mm) that allow rapid, non-equilibrium flow of water and dissolved substances (Jarvis, 2007; White, 1985). Additionally, strongly sorbing solutes such as pesticides and P have been observed to leach more readily in structured soils than in sieved soils due to their tendency to sorb onto mobile colloids (de Jonge et al., 2004; Larsson and Jarvis, 2000; Paradelo et al., 2013). These colloids are efficiently filtered in matrix flow within sieved soils but are readily transported via macropore pathways in structured soils (Jacobsen et al., 1997; Jarvis, 2007). It is possible that such preferential transport of alkalinity did happen in this study, but bulk measurements of soil pH and DOC concentrations failed to detect localized effects. Figure 2 reveals regions of elevated soil pH (dark blue) in untreated zones of lime and lime + OA treatments of the intact soil, suggesting the presence of preferential flow in macropores. Such effects, though minor, could hold substantial implications in field conditions where plant roots actively exploit macropores for water and nutrient uptake (Atkinson et al., 2020; Colombi et al., 2017). Yet, the possibility of imaging artifacts due to incomplete sample–sensor contact in Fig. 2 cannot be excluded. Two possible explanations are given for the lack of pronounced preferential flow observed in that case. First, the applied irrigation rate, averaging 2 mm d−1, may have been insufficient to generate non-equilibrium flow in soil macropores. The literature indicates that irrigation intensities exceeding approximately 1 mm h−1 are typically required to activate such flows (Beven and Germann, 1982; Jarvis, 2007). Higher rates could not be achieved in this experiment, as excessive irrigation risked overflowing the columns. This risk increased further by potential clogging of the porous ceramic plates at the base of the columns. Second, the Retisol used may be low-structured, with limited macropore presence and weak pore connectivity. Water primarily moves through the soil matrix in such soils, exposing solutes to a larger surface area and increasing interaction with soil particles compared to preferential flow in structured soils. This leads to more adsorption and dispersion of solutes, which is aggravated by the high retention time of water in low-structured soils (Jarvis, 2007; Norgaard et al., 2013).
This study confirms that combining lime with an organic amendment can enhance alkalinity leaching to the subsoil compared to a lime-only treatment, yet the success of this process depends on soil properties. Specifically, highly weathered soils, such as the Vietnamese Ferralsol, show limited DOC-mediated alkalinity transport due to strong DOC adsorption, which likely prevents lime-derived Ca2+ from reaching the subsoil. These results suggest that DOC-mediated alkalinity leaching is unlikely to occur in highly weathered tropical soils. This is a challenging outcome for acid-soil management in these regions, where low-cost liming solutions are needed. Contrary to our hypothesis, alkalinity leaching was not more pronounced in structured soils than in sieved soils. While preferential flow through macropores has been shown to promote chemical leaching in structured soils, this effect was not observed in our study, potentially because local effects remained undetected by bulk measurements or due to limited irrigation rates and/or low macropore connectivity in the Retisol. Our results underscore the need for further research into the complex interactions of soil chemistry, structure, and hydrology that govern alkalinity leaching, especially in field conditions where macropore flow and root uptake may alter the transport dynamics of organic anions and lime.
The software that was used is the program Visual Minteq, which is openly accessible and included in the reference list (https://vminteq.com/, Gustafsson, 2000).
Data will be made available by the corresponding author upon reasonable request.
The supplement related to this article is available online at https://doi.org/10.5194/soil-11-899-2025-supplement.
HVR: conceptualization, methodology, formal analysis, investigation, writing – original draft, visualization. LVA: formal analysis, investigation. TvD: conceptualization, methodology, writing – review and editing, supervision. ES: conceptualization, methodology, writing – review and editing, supervision, funding acquisition.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors. The views and opinions expressed are those of the authors only and do not necessarily reflect those of the European Union or the European Research Council Executive Agency. Neither the European Union nor the granting authority can be held responsible.
Declaration of generative AI and AI-assisted technologies in the writing process: during the preparation of this work, the author(s) used ChatGPT in order to improve their writing style on an earlier version of the paper. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
This research has been supported by the European Research Council, EU HORIZON EUROPE European Research Council (grant-no. 101054917).
This paper was edited by Luisella Celi Celi and reviewed by two anonymous referees.
Amery, F., Degryse, F., Van Moorleghem, C., Duyck, M., and Smolders, E.: The dissociation kinetics of Cu-dissolved organic matter complexes from soil and soil amendments, Anal. Chim. Acta, 670, 24–32, https://doi.org/10.1016/j.aca.2010.04.047, 2010.
Atkinson, J. A., Hawkesford, M. J., Whalley, W. R., Zhou, H., and Mooney, S. J.: Soil strength influences wheat root interactions with soil macropores, Plant Cell Environ., 43, 235–245, https://doi.org/10.1111/pce.13659, 2020.
Azam, G. and Gazey, C.: Slow movement of alkali from surface-applied lime warrants the introduction of strategic tillage for rapid amelioration of subsurface acidity in south-western Australia, Soil Res., 59, 97–106, https://doi.org/10.1071/SR19329, 2020.
Bergen, B., Moens, C., De Winter, A., Ricou, F., and Smolders, E.: Colloids facilitate cadmium and uranium transport in an undisturbed soil: A comparison of soil solution isolation methods, Sci. Total Environ., 890, 164419, https://doi.org/10.1016/j.scitotenv.2023.164419, 2023.
Beven, K. and Germann, P.: Macropores and water flow in soils, Water Resour. Res., 18, 1311–1325, https://doi.org/10.1029/WR018i005p01311, 1982.
Butterly, C. R., Costello, B., Lauricella, D., Sale, P., Li, G., and Tang, C.: Alkalinity movement down acid soil columns was faster when lime and plant residues were combined than when either was applied separately, Eur. J. Soil Sci., 72, 313–325, https://doi.org/10.1111/ejss.12945, 2021.
Colombi, T., Braun, S., Keller, T., and Walter, A.: Artificial macropores attract crop roots and enhance plant productivity on compacted soils, Sci. Total Environ., 574, 1283–1293, https://doi.org/10.1016/j.scitotenv.2016.07.194, 2017.
Conyers, M. K. and Scott, B. J.: The influence of surface incorporated lime on subsurface soil acidity, Aust. J. Exp. Agric., 29, 201–207, https://doi.org/10.1071/ea9890201, 1989.
de Jonge, L. W., Moldrup, P., Rubæk, G. H., Schelde, K., and Djurhuus, J.: Particle Leaching and Particle-Facilitated Transport of Phosphorus at Field Scale, Vadose Zone J., 3, 462–470, https://doi.org/10.2136/vzj2004.0462, 2004.
George, E., Horst, W. J., and Neumann, E.: Chapter 17 – Adaptation of Plants to Adverse Chemical Soil Conditions, in: Marschner's Mineral Nutrition of Higher Plants, edited by: Marschner, P., 3rd edn., Academic Press, San Diego, 409–472, https://doi.org/10.1016/B978-0-12-384905-2.00017-0, 2012.
Gustafsson, J. P.: Visual MINTEQ version 3.1, KTH Royal Institute of Technology [code], https://vminteq.com/ (last access: 27 November 2024), 2024.
Haynes, R. J. and Judge, A.: Influence of surface-applied poultry manure on topsoil and subsoil acidity and salinity: A leaching column study, J. Plant Nutr. Soil Sci., 171, 370–377, https://doi.org/10.1002/jpln.200700167, 2008.
Haynes, R. J. and Mokolobate, M. S.: Amelioration of Al toxicity and P deficiency in acid soils by additions of organic residues: a critical review of the phenomenon and the mechanisms involved, Nutr. Cycl. Agroecosystems, 59, 47–63, https://doi.org/10.1023/A:1009823600950, 2001.
Herbert, B. E. and Bertsch, P. M.: Characterization of Dissolved and Colloidal Organic Matter in Soil Solution: A Review, in: Carbon Forms and Functions in Forest Soils, John Wiley & Sons, Ltd, 63–88, https://doi.org/10.2136/1995.carbonforms.c5, 1995.
Hue, N. V. and Licudine, D. L.: Amelioration of Subsoil Acidity through Surface Application of Organic Manures, J. Environ. Qual., 28, 623–632, https://doi.org/10.2134/jeq1999.00472425002800020028x, 1999.
Jacobsen, O. H., Moldrup, P., Larsen, C., Konnerup, L., and Petersen, L. W.: Particle transport in macropores of undisturbed soil columns, J. Hydrol., 196, 185–203, https://doi.org/10.1016/S0022-1694(96)03291-X, 1997.
Jardine, P. M., McCarthy, J. F., and Weber, N. L.: Mechanisms of Dissolved Organic Carbon Adsorption on Soil, Soil Sci. Soc. Am. J., 53, 1378–1385, https://doi.org/10.2136/sssaj1989.03615995005300050013x, 1989.
Jarvis, N. J.: A review of non-equilibrium water flow and solute transport in soil macropores: principles, controlling factors and consequences for water quality, Eur. J. Soil Sci., 58, 523–546, https://doi.org/10.1111/j.1365-2389.2007.00915.x, 2007.
Kaiser, K., Guggenberger, G., and Zech, W.: Sorption of DOM and DOM fractions to forest soils, Geoderma, 74, 281–303, https://doi.org/10.1016/S0016-7061(96)00071-7, 1996.
Kalbitz, K., Solinger, S., Park, J.-H., Michalzik, B., and Matzner, E.: Controls on the Dynamics of Dissolved Organic Matter in Soils: A Review, Soil Sci., 165, 277–304, https://doi.org/10.1097/00010694-200004000-00001, 2000.
Kindler, R., Siemens, J., Kaiser, K., Walmsley, D., Bernhofer, C., Buchmann, N., Cellier, P., Eugster, W., Gleixner, G., Grünwald, T., Heim, A., Ibrom, A., Jones, S., Jones, M., Klumpp, K., Kutsch, W. L., Larsen, K., Lehuger, S., Loubet, B., and Kaupenjohann, M.: Dissolved carbon leaching from soil is a crucial component of the net ecosystem carbon balance, Glob. Change Biol., 17, 1167–1185, https://doi.org/10.1111/j.1365-2486.2010.02282.x, 2011.
Kochian, L., Hoekenga, O., and Piñeros, M.: How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency, Annu. Rev. Plant Biol., 55, 459–493, https://doi.org/10.1146/annurev.arplant.55.031903.141655, 2004.
Kögel-Knabner, I. and Amelung, W.: 12.7 – Dynamics, Chemistry, and Preservation of Organic Matter in Soils, in: Treatise on Geochemistry, edited by: Holland, H. D. and Turekian, K. K., 2nd edn., Elsevier, Oxford, 157–215, https://doi.org/10.1016/B978-0-08-095975-7.01012-3, 2014.
Kreuzeder, A., Santner, J., Scharsching, V., Oburger, E., Hoefer, C., Hann, S., and Wenzel, W.: In situ observation of localized, sub-mm scale changes of phosphorus biogeochemistry in the rhizosphere, Plant Soil, 424, 1–17, https://doi.org/10.1007/s11104-017-3542-0, 2018.
Lægdsmand, M., Villholth, K. G., Ullum, M., and Jensen, K. H.: Processes of colloid mobilization and transport in macroporous soil monoliths, Geoderma, 93, 33–59, https://doi.org/10.1016/S0016-7061(99)00041-5, 1999.
Larsson, M. H. and Jarvis, N. J.: Quantifying interactions between compound properties and macropore flow effects on pesticide leaching, Pest Manag. Sci., 56, 133–141, https://doi.org/10.1002/(SICI)1526-4998(200002)56:2<133::AID-PS103>3.0.CO;2-N, 2000.
Lauricella, D., Butterly, C. R., Weng, Z. (Han), Clark, G. J., Sale, P. W. G., Li, G., and Tang, C.: Impact of novel materials on alkalinity movement down acid soil profiles when combined with lime, J. Soils Sediments, 21, 52–62, https://doi.org/10.1007/s11368-020-02747-4, 2021.
Lewis, J. and Sjöstrom, J.: Optimizing the experimental design of soil columns in saturated and unsaturated transport experiments, J. Contam. Hydrol., 115, 1–13, https://doi.org/10.1016/j.jconhyd.2010.04.001, 2010.
Li, G. D., Conyers, M. K., Helyar, K. R., Lisle, C. J., Poile, G. J., and Cullis, B. R.: Long-term surface application of lime ameliorates subsurface soil acidity in the mixed farming zone of south-eastern Australia, Geoderma, 338, 236–246, https://doi.org/10.1016/j.geoderma.2018.12.003, 2019.
Liu, J. and Hue, N.: Amending subsoil acidity by surface applications of gypsum, lime, and composts, Commun. Soil Sci. Plant Anal., 32, 2117–2132, https://doi.org/10.1081/CSS-120000273, 2001.
Marschner, H.: Mechanisms of adaptation of plants to acid soils, in: Plant-Soil Interactions at Low pH: Proceedings of the Second International Symposium on Plant-Soil Interactions at Low pH, 24–29 June 1990, Beckley West Virginia, USA, edited by: Wright, R. J., Baligar, V. C., and Murrmann, R. P., Springer Netherlands, Dordrecht, 683–702, https://doi.org/10.1007/978-94-011-3438-5_78, 1991.
Miyazawa, M., Pavan, A., and Franchini, J.: Evaluation of Plant Residues on the Mobility of Surface Applied Lime, Braz. Arch. Biol. Technol., 45, https://doi.org/10.1590/S1516-89132002000300001, 2002.
Moore, T. R., de Souza, W., and Koprivnjak, J.-F.: Controls on the sorption of dissolved organic carbon by soils, Soil Sci., 154, 120, 1992.
Norgaard, T., Moldrup, P., Olsen, P., Vendelboe, A. L., Iversen, B. V., Greve, M. H., Kjaer, J., and de Jonge, L. W.: Comparative Mapping of Soil Physical–Chemical and Structural Parameters at Field Scale to Identify Zones of Enhanced Leaching Risk, J. Environ. Qual., 42, 271–283, https://doi.org/10.2134/jeq2012.0105, 2013.
Paradelo, M., Moldrup, P., Arthur, E., Naveed, M., Holmstrup, M., López-Periago, J. E., and de Jonge, L. W.: Effects of Past Copper Contamination and Soil Structure on Copper Leaching from Soil, J. Environ. Qual., 42, 1852–1862, https://doi.org/10.2134/jeq2013.05.0209, 2013.
Renneson, M., Vandenberghe, C., Dufey, J., Marcoen, J. M., Bock, L., and Colinet, G.: Degree of phosphorus saturation in agricultural loamy soils with a near-neutral pH, Eur. J. Soil Sci., 66, 33–41, https://doi.org/10.1111/ejss.12207, 2015.
Santner, J., Larsen, M., Kreuzeder, A., and Glud, R.: Two decades of chemical imaging of solutes in sediments and soils – A review, Anal. Chim. Acta, 878, 9–42, https://doi.org/10.1016/j.aca.2015.02.006, 2015.
Schwertmann, U.: Solubility and dissolution of iron oxides, Plant Soil, 130, 1–25, https://doi.org/10.1007/BF00011851, 1991.
Siemens, J., Ilg, K., Lang, F., and Kaupenjohann, M.: Adsorption controls mobilization of colloids and leaching of dissolved phosphorus, Eur. J. Soil Sci., 55, 253–263, https://doi.org/10.1046/j.1365-2389.2004.00596.x, 2004.
Smith, C. J., Goh, K. M., Bond, W. J., and Freney, J. R.: Effects of organic and inorganic calcium compounds on soil-solution pH and aluminium concentration, Eur. J. Soil Sci., 46, 53–63, https://doi.org/10.1111/j.1365-2389.1995.tb01812.x, 1995.
Sumner, M. E., Shahandeh, H., Bouton, J., and Hammel, J.: Amelioration of an Acid Soil Profile through Deep Liming and Surface Application of Gypsum, Soil Sci. Soc. Am. J., 50, 1254–1258, https://doi.org/10.2136/sssaj1986.03615995005000050034x, 1986.
Tang, C., Asseng, S., Diatloff, E., and Rengel, Z.: Modelling yield losses of aluminium-resistant and aluminium-sensitive wheat due to subsurface soil acidity: effects of rainfall, liming and nitrogen application, Plant Soil, 254, 349–360, https://doi.org/10.1023/A:1025597905001, 2003.
Tang, C., Weligama, C., and Sale, P.: Subsurface Soil Acidification in Farming Systems: Its Possible Causes and Management Options, in: Molecular Environmental Soil Science, edited by: Xu, J. and Sparks, D. L., Springer Netherlands, Dordrecht, 389–412, https://doi.org/10.1007/978-94-007-4177-5_13, 2013.
Tipping, E.: The adsorption of aquatic humic substances by iron oxides, Geochim. Cosmochim. Ac., 45, 191–199, https://doi.org/10.1016/0016-7037(81)90162-9, 1981.
Tschiersch, H., Liebsch, G., Stangelmayer, A., Borisjuk, L., and Rolletschek, H.: Planar Oxygen Sensors for Non Invasive Imaging in Experimental Biology, https://doi.org/10.5772/17893, 2011.
van der Watt, H. v. H., Barnard, R. O., Cronje, I. J., Dekker, J., Croft, G. J. B., and van der Walt, M. M.: Amelioration of subsoil acidity by application of a coal-derived calcium fulvate to the soil surface, Nature, 350, 146–148, https://doi.org/10.1038/350146a0, 1991.
Verbeeck, M., Hiemstra, T., Thiry, Y., and Smolders, E.: Soil organic matter reduces the sorption of arsenate and phosphate: a soil profile study and geochemical modelling, Eur. J. Soil Sci., 68, 678–688, https://doi.org/10.1111/ejss.12447, 2017.
von Uexküll, H. R. and Mutert, E.: Global extent, development and economic impact of acid soils, Plant Soil, 171, 1–15, https://doi.org/10.1007/BF00009558, 1995.
Werts, S.: Factors of soil formation: Soil biota and organic matter as a factor for soil formation and weathering processes, in: Encyclopedia of Soils in the Environment, edited by: Goss, M. J. and Oliver, M., 2nd edn., Academic Press, Oxford, 25–33, https://doi.org/10.1016/B978-0-12-822974-3.00070-7, 2023.
White, R. E.: The Influence of Macropores on the Transport of Dissolved and Suspended Matter Through Soil, in: Advances in Soil Science, New York, NY, 95–120, https://doi.org/10.1007/978-1-4612-5090-6_3, 1985.
Wong, M. and Swift, R.: Role of Organic Matter in Alleviating Soil Acidity, in: Handbook of Soil Acidity, edited by: Rengel, Z., Marcel Dekker, New York, 337–358, ISBN 0824708903, 2003.
Wright, R. J., Hern, J. L., Baligar, V. C., and Bennett, O. L.: The effect of surface applied soil amendments on barley root growth in an acid subsoil, Commun. Soil Sci. Plant Anal., 16, 179–192, https://doi.org/10.1080/00103628509367595, 1985.
Yan, F., Schubert, S., and Mengel, K.: Soil pH changes during legume growth and application of plant material, Biol. Fertil. Soils, 23, 236–242, https://doi.org/10.1007/BF00335950, 1996.




