the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Contribution of soil microbial necromass carbon to soil organic carbon fractions and its influencing factors in different grassland types
Shenggang Chen
Yaqi Zhang
Jun Ma
Mingyue Bai
Jinxiao Long
Ming Liu
Yinglong Chen
Jianbin Guo
Lin Chen
Microbial necromass carbon (MNC) is a significant source of soil organic carbon (SOC). However, the contribution of microbial necromass to different organic carbon fractions and their influencing factors in various soil layers under different grassland types remains unclear. This study was conducted through a comprehensive investigation of soil profiles (0–20, 20–40, and 40–100 cm) across four grassland types in Ningxia, China, encompassing meadow steppe, typical steppe, desert steppe, and steppe desert. We quantified mineral-associated organic carbon (MAOC), particulate organic carbon (POC), and their respective microbial necromass components, including total microbial necromass carbon (TNC), fungal necromass carbon (FNC), and bacterial necromass carbon (BNC), and analyzed the contributions to SOC fractions and influencing factors. Our findings reveal three key insights. First, the contents of MAOC and POC in the 0–100 cm soil layer were in the following order of magnitude: Meadow steppe > Typical steppe > Desert steppe > Steppe desert, with the average content of POC being 9.3 g kg−1, which was higher than the average content of MAOC (8.73 g kg−1). Second, the content of microbial TNC in MAOC and POC decreased with soil depth, the average content of FNC was 3.02 and 3.85 g kg−1, which were higher than the average content of BNC (1.64 and 2.08 g kg−1). FNC dominated both MAOC and POC, and its contribution was higher than the contribution of BNC. Third, through regression analysis and random forest modeling, we identified key environmental drivers of MNC dynamics: mean annual rainfall, electrical conductance, and soil total nitrogen emerged as primary regulators in surface soils (0–20 cm), while available potassium, SOC, and mean annual temperature dominated deeper soil layers (20–100 cm). This research contributes by: (1) establishing the vertical distribution patterns of MNC and SOC fractions in soil profiles; (2) quantifying the relative contributions of MNC to SOC fractions across different grassland ecosystems soil profiles and elucidating their environmental controls, offers a deeper understanding of the mechanisms driving MNC accumulation in SOC fractions in diverse grassland ecosystems, and providing data support for further research on the microbiological mechanisms of soil organic carbon formation and accumulation in arid and semi-arid regions.
- Article
(2751 KB) - Full-text XML
-
Supplement
(824 KB) - BibTeX
- EndNote
Soil organic carbon (SOC) is the largest carbon reservoir in grassland ecosystems and is strongly influenced by vegetation and microorganisms under certain conditions (Lehmann and Kleber, 2015). Microbial necromass carbon (MNC) is a crucial source of SOC, and its formation and accumulation processes vary significantly across different grassland types and SOC fractions, leading to varying contributions to SOC (Deng and Liang, 2022). The partitioning of SOC into particulate organic carbon (POC) and mineral-associated organic carbon (MAOC) provides critical insights into carbon stabilization mechanisms. While POC primarily originates from plant residues, with a high carbon: nitrogen ratio and rapid turnover rate, and represents the more labile carbon pool, MAOC is mainly derived from microbial sources, with a slower turnover rate, allowing it to persist in the soil for centuries, thus playing a vital role in long-term SOC stabilization (Angst et al., 2021; Wang et al., 2021b). The proportion of microbial and plant-derived necromass carbon varies due to microbial decomposition processes (Bölscher et al., 2024), leading to significant differences in the contribution of microbial-derived carbon to MAOC and POC formation. Recent advances in soil organic matter research have revealed that microbial-derived carbon contributes approximately 52 % to MAOC, while plant-derived carbon contributes about 40 % to POC (Liang et al., 2019). These results underscore the necessity to investigate the MNC contribution to SOC fractions, which is fundamental for accurately evaluating the environmental benefits and carbon sequestration potential of ecological conservation initiatives (Hou et al., 2024).
The accumulation of MNC in grassland ecosystems is governed by a complex interplay of biotic and abiotic factors (Li et al., 2017). Variations in plant biomass and diversity across different grassland types significantly influence soil physicochemical properties and microbial community structure, while land use patterns, soil depth, soil nutrients, and climatic conditions further influence the accumulation of MNC (Yang et al., 2024). Recent studies have demonstrated the substantial contribution of MNC to SOC pools, Wang et al. (2021a) found that nearly 47 % contributes in the 0–20 cm soil layer of grasslands. Cotrufo et al. (2019) demonstrated that MAOC contributes over 50 % to SOC accumulation in grasslands, highlighting its critical role in carbon stabilization. Notably, He et al. (2022) observed that the accumulation of MNC in alpine grasslands is closely related to soil depth. Liao et al. (2023) found that necromass carbon content in the 0–5 and 5–20 cm soil layers of grasslands on the Loess Plateau ranged from 0.69 to 16.41 g kg−1. Additionally, drought thresholds and soil stoichiometric ratios are critical factors influencing MNC accumulation in grasslands (Hao et al., 2021). Dou et al. (2023) highlighted that MNC is stored more in the MAOC fraction across different grassland types and soil layers, with soil bulk density, pH, and total organic carbon being the primary factors influencing its contribution to SOC accumulation.
However, our understanding of MNC dynamics remains incomplete, most studies focus on the 0–20 and 20–40 cm soil layers, with limited research on MNC in deeper soil layers (> 60 cm), this knowledge gap is particularly pronounced in ecologically transitional zones (An et al., 2010; Du et al., 2021). Particular in Ningxia, which is one of the three pilot provinces of “Research on climate change adaptation in China”, and encompasses diverse grassland types representative of northern Chinese ecosystems: meadow steppe, typical steppe, desert steppe and steppe desert. While previous research in Ningxia has primarily have focused on conventional SOC parameters (e.g., soil carbon density, storage, and spatial distribution of water-soluble organic carbon), regarding the dynamics of MAOC and POC fractions, particularly the contribution of MNC to their accumulation in deeper soil layers (> 60 cm) are not yet well understood (Wu et al., 2025). To fill this gap, our research focuses on four different grassland types, (1) investigate the vertical distribution (0–100 cm) of SOC fractions and MNC across different grassland types; (2) identify the relative contribution of fungi and bacteria to SOC fractions; (3) elucidate the key drivers influencing MNC contribution to MAOC and POC accumulation in deeper soil layers. We hypothesized that: (1) the contents of SOC fractions and MNC would be decreased with soil deep; (2) The contribution of fungal necromass carbon is higher than that of bacteria; (3) The key factors influencing MNC contribution to MAOC and POC accumulation are mean annual precipitation and soil total nitrogen. By elucidating the microbial mechanisms of SOC formation and accumulation in different grassland types and provides crucial insights for optimizing grassland management strategies and supporting regional carbon neutrality objectives, at the same time, it provides a theoretical basis and data support for the realization of the regional “dual-carbon” goal.
2.1 Study Area
The study area (35°14′–39°23′ N, 104°17′–107°39′ E) is located in the Ningxia Hui Autonomous Region, China, with a total area of 66 400 km2, represents a transitional zone between the Loess Plateau and the Mongolian Plateau (Ji et al., 2023). Ningxia belongs to typical continental semi-humid semi-arid climate, and hosting a remarkable diversity of grassland ecosystems that cover 47 % of its land area – encompassing nearly all major grassland types found in northern China. In the southern Loess Plateau, meadow steppe (MS) and typical steppe (TS) dominate, with the TS concentrated near Guyuan City (e.g., Yunwu Mountain Grassland Nature Reserve). This area belonging to a semi-arid climate, with annual precipitation generally ranging from 300 to 400 mm, and is dominated by drought-resistant perennial tufted grasses, the soil is dominated by black clay. In contrast, MS is mainly distributed on the shady slopes and valleys of Liupan Mountains and other mountainous areas, where water conditions are better, the climate is more humid, and annual precipitation is generally around 400–600 mm. MS consists of perennial perennial and rhizomatous grasses in the middle of the arid zone, the soils are mainly mountain brown loam, mountain gray-brown soil and black clay. Desert steppe (DS) is distributed in the central and northern parts of Ningxia, representing a transitional zone between grassland and desert. It has an arid climate, with annual precipitation generally around 200–300 mm, and vegetation cover of 40 %–60 %, and is dominated by dry perennial grasses with participation of small dry shrubs. Steppe desert (SD) is distributed in the northern and northwestern parts of Ningxia, adjacent to the Tengger Desert and Mao Wusu Desert. The climate is extremely arid, annual precipitation is usually less than 200 mm, vegetation is sparse (< 30 %), and super-arid shrubs and small half-shrubs dominate (Zhang et al., 2025, 2023). The soil is predominantly light gray calcareous in DS and SD. This diversity offers a unique natural laboratory to investigate how varying ecosystems respond to climatic shifts.
2.2 Site Selection and Soil Sampling
To capture the ecological diversity along the precipitation gradient from south to north, four grassland types were selected: MS, TS, DS, and SD (Fig. 1). The number of sampling sites for each grassland type was proportional to their respective areas: MS (5 sites), DS (7 sites), TS (5 sites), and SD (5 sites). The latitude, longitude, and elevation of each site were recorded, and mean annual temperature (MAT) and annual precipitation (MAP) were obtained from the databases (http://www.worldclim.org/, last access: 30 September 2024). At each site, three 20 × 20 m plots were established, with a minimum distance of 20 m between plots. Soil samples were collected from 0–20 cm, 20–40, and 40–100 cm layers using a soil auger, mixed thoroughly, and air-dried in the laboratory (An et al., 2010; Wu et al., 2025). After removing plant roots and gravel, the soil was sieved through 2 and 0.15 mm meshes for MAOC, POC, and soil physicochemical property analyses. Additionally, 4–5 g of soil was reserved for amino sugar analysis. Vegetation surveys were conducted in three randomly selected 1 × 1 m subplots within each plot (Table S1 in the Supplement).
2.3 Measurement Methods
2.3.1 Soil Physicochemical Properties
Soil bulk density (BD) was determined using the core methodwith a 100 cm3 ring knife (5 cm height, 5.05 cm diameter) (Wang et al., 2022b). Soil water content (SWC) was assessed via the oven-drying method, where fresh soil was dried at 102 °C until a constant weight was achieved (Li et al., 2018). Soil pH was measured using a pH meter (pHS-3C) with a soil-to-water ratio of 1:2.5 () (Roberts et al., 2007). SOC was quantified using the K2Cr2O7 external heating method, followed by titration with 0.1 M FeSO4 (Ding et al., 2019). Total nitrogen (TN) was determined using the Kjeldahl method using the Kjeltec 8400 (FOSS, Denmark). Available nitrogen (AN) using alkaline hydrolysis diffusion. Total phosphorus (TP) was measured by an ultraviolet spectrophotometer (UV3200, Shimadu Corporation, Japan) after wet digestion with H2SO4 and HClO4. Available phosphorus (AP) using sodium bicarbonate extraction. Available potassium (AK) was extract with 1 mol L−1 ammonium acetate solution at pH 7.0 and subsequent determination by atomic absorption and emission spectrophotometry. Total carbon (TC) was analyzed using the potassium dichromate external heating method (Chai et al., 2024; Zhang et al., 2021). Soil electrical conductivity (EC) was measured using a conductivity meter.
2.3.2 MAOC and POC Measurement
The separation into the coarse fraction and mineral-associated fraction was achieved using the density-gravity method. Specifically, 20.00 g of air-dried soil (passed through a 2 mm sieve) was weighed into a conical flask, followed by the addition of 60 mL of sodium hexametaphosphate solution (5 %, ). The mixture was shaken on an orbital shaker for 18 h (25 °C, 180 r min−1), after which the suspension was passed through a 53 µm nylon sieve and rinsed with distilled water until the effluent became clear. The separated samples were oven-dried at 60 °C and ground. The coarse fraction (particle size > 53 µm) was designated as particulate organic matter, while the fine fraction (particle size < 53 µm) was classified as mineral-associated organic matter. The organic carbon content in each fraction was determined using the potassium dichromate-external heating method (Sokol et al., 2019b). POC and MAOC were calculated according to Eqs. (1) and (2), with units expressed in g kg−1.
Where ΔM1 represents the oven-dry weight of the upper-layer soil sample after separation (g); ΔM2 denotes the oven-dry weight of the lower-layer soil sample after separation (g); M is the total mass of the soil sample before separation (g); CPOM refers to the organic carbon content of the upper-layer soil sample determined by the potassium dichromate-external heating method (g kg−1); and CMAOM measured indicates the organic carbon content of the lower-layer soil sample determined by the potassium dichromate-external heating method (g kg−1).
2.3.3 Amino Sugar Measurement
Amino sugars were measured according to the method described by Indorf et al. (2011). Soil samples underwent hydrolysis, purification, and derivatization, followed by gas chromatography (GC) analysis to determine four amino sugar derivatives: glucosamine (GlcN), mannosamine (ManN), galactosamine (GalN), and muramic acid (MurA). TNC was calculated using the optimized formulas by Hua et al. (2024):
Where FNC is fungal necromass carbon, BNC is bacterial necromass carbon, TNC is total necromass carbon, and GlcN and MurA are the concentrations of glucosamine and muramic acid in the soil, respectively. The molecular weights of GlcN and MurA are 179.17 and 251.23, respectively, and 31.3 is the conversion factor for bacterial muramic acid to bacterial necromass carbon (Liang et al., 2019).
2.4 Data Analysis
Before analysis, all variables were tested for normality and homogeneity of variances, and log-transformations were performed when necessary. Data were organized using Excel 2023 and Word 2023 and statistical calculations (i.e., correlations and significant differences) were conducted using the SPSS 20.0 statistical software package (SPSS Inc, Chicago, USA). One-way, two-way ANOVA and LSD tests were used to assess the differences of soil physicochemical properties, SOC fractions, MNC among the different sampling sites, correlation analysis was considered significant at p<0.05. The relationship between BNC and FNC, and , and were analyzed by univariate linear regression. The use of Principal component analysis to show that the soil properties of different grassland types were significantly different between the 0–20 and the 20–40 and 40–100 cm soil layers. And using Spearman correlation to analysis the relationship between environmental variables and MAOC, , , TNC/MAOC, POC, , , . Subsequently, we used a random forest model to predict the influences affecting the accumulation of FNC, BNC, and TNC in different soil layers of different grassland types. The random forest modeling were conducted using R (version 4.3.1), with packages including “ggplot2”, “tidyverse”, “randomForest”, “rfUtilities”, and “rfpermute” (Liao et al., 2023).
3.1 Soil Physicochemical Properties Across Different Grassland Types
Significant vertical variations in soil properties were observed across the 0–100 cm soil profile among different grassland types (Fig. S1). In MS and TS, the 0–20 cm layer showed significantly higher SWC than the deep layers (20–40 and 40–100 cm). In contrast, DS and SD displayed an inverse trend, with lower SWC in the upper layers. SOC and TN were markedly higher in MS and TS than in SD and DS, with no significant vertical differences in DS and SD (p>0.05). Both TC and AK differed significantly between the 0–20 and 40–100 cm layers across all grassland types, although no differences were found between the 20–40 and 40–100 cm layers. Notably, TC was lowest in the 0–20 cm layer, whereas AK peaked in this layer. BD and available AP showed little variation, whereas EC varied significantly within the same soil layer across different grassland types.
3.2 Variations in MAOC and POC content across different grassland types
MAOC and POC contents were highest in MS, followed by TS and DS, with SD having the lowest contents. MAOC decreased with depth in all grasslands except DS (Fig. 2a). In MS and DS, POC content' was higher in the 20–40 cm layer than in the 0–20 cm layer, with significant differences among the three soil layers (p<0.05, Fig. 2b). In the 0–20 cm layer, MAOC differed significantly among grassland types, except between SD and HM (p<0.05, Fig. 2a). In the 20–40 and 40–100 cm layers, both MAOC and POC varied significantly across grassland types. In HM, MAOC was higher in the 20–40 cm layer than in the 0–20 cm layer, whereas POC showed the opposite trend. The 40–100 cm layer consistenly had the lowest MAOC and POC contents across all grassland types.
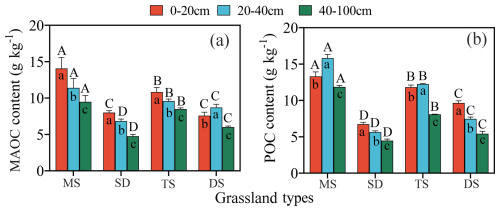
Figure 2Contents of MAOC (a) and POC (b) in 0–100 cm soil layers under different grassland types. Different uppercase letters indicate significant differences in different grassland types in the same soil layer, and different lowercase letters indicate significant differences in different soil layers under the same grassland types (p<0.05). MAOC: mineral-associated organic carbon; POC: particulate organic carbon. The bars in red, blue and green represent the soil layers 0–20, 20–40 and 40–100 cm respectively. MS: meadow steppe; SD: steppe desert; TS: typical steppe; DS: desert steppe.
3.3 Characteristics of changes in MNC and proportion of MAOC and POC in different grassland types
3.3.1 Characterization of changes in the content and proportion of MNC in MAOC
The ratio, which reflects the relative contributions of fungi and bacteria to MNC, demonstrated significant positive correlations across all soil layers (0–20 cm: R2 = 0.79, p<0.0001; 20–40 cm: R2 = 0.63, p<0.0001; 40–100 cm: R2=0.43, p<0.001, Fig. S2a). SD consistently had the highest ratio (p<0.05, Fig. S2b). FNC contributed 2.65–4.63 times more than BNC in the 0–20 cm layer, 2.06–10.17 times in the 20–40 cm layer, and 2.30–8.03 times in the 40–100 cm layer (Fig. S2).
In the 0–20 cm soil layer, BNC, FNC, and TNC within MAOC ranged from 1.5–4.0, 2.2–6.6, and 3.5–10.6 g kg−1, respectively. These values were significantly higher than those in deeper layers (p<0.05, Fig. 3). In the 20–40 cm layer, SD exhibited the lowest BNC content (0.43 g kg−1) (Fig. 3a), while DS recorded the lowest FNC content (1.69 g kg−1) (Fig. 3b). The TNC content across the 0–100 cm layer followed the order: MS > TS > DS > SD, with MS significantly higher than other grassland types (p<0.05, Fig. 3c).
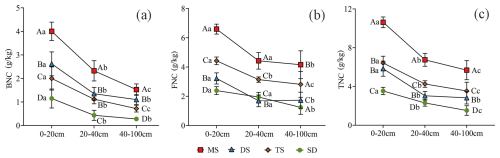
Figure 3Contents of BNC (a) FNC (b) and TNC (c) in MAOC in different soil layers under different grassland types. Different uppercase letters indicate significant differences in different grassland types in the same soil layer (p<0.05), and different lowercase letters indicate significant differences in different soil layers under the same grassland types (p<0.05). The red square, blue triangle, brown rhombus and green circle represent: meadow steppe (MS); desert steppe (DS); typical steppe (TS); steppe desert (SD). FNC: fungal necromass carbon, BNC: bacterial necromass carbon, TNC: total necromass carbon.
In the 0–20 cm layer, DS exhibited the highest ratio (35 %) followed by MS (28 %), TS (18 %), and SD (14 %) (p<0.05, Fig. 4a). The ratio for DS (42 %) was lower than MS (48 %) but higher than in TS (41 %) and SD (29 %). SD differed significantly from other grassland types (p<0.05, Fig. 4b). The ratios in DS and MS (77 %) exceeded, those in TS (66 %) and SD (44 %) (p<0.05, Fig. 4c). In the 20–40 cm layer, MS had a higher ratio (21 %) than DS (15 %), TS (12 %), and SD (5 %) (p<0.05, Fig. 4a). The 40–100 cm layer exhibited trends similar to those in the 0–20 cm layer. Contributions of and were positively correlated in the 0–20 cm layer (R2 = 0.36, p<0.001), but no significant correlations were found in the 20–40 and 40–100 cm layers (R2 = 0.03 and 0.05, respectively, p>0.05, Fig. 4d).
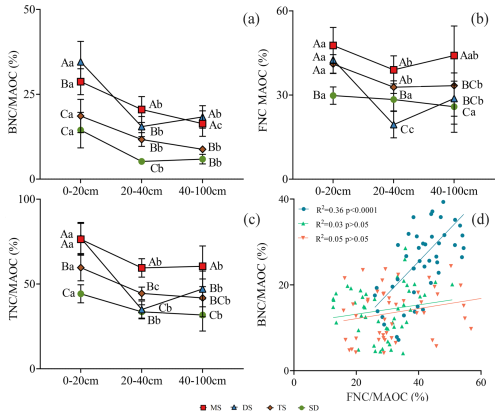
Figure 4The contribution of BNC, FNC and TNC in MAOC in different soil layers under different grassland types. (a) The contribution of BNC to MAOC; (b) the contribution of FNC to MAOC; (c) the contribution of TNC to MAOC. Different uppercase letters indicate significant differences in different grassland types in the same soil layer (p<0.05), and different lowercase letters indicate significant differences in different soil layers under the same grassland types (p<0.05). The red square, blue triangle, brown rhombus and green circle represent: meadow steppe (MS); desert steppe (DS); typical steppe (TS); steppe desert (SD). (d) the relationship between and . The blue circle, green triangle and orange inverted triangle represent the soil layers 0–20, 20–40 and 40–100 cm respectively. FNC: fungal necromass carbon, BNC: bacterial necromass carbon, MAOC: mineral-associated organic carbon.
3.3.2 Characterization of changes in the content and proportion of MNC in POC
In the 0–100 cm soil layer, the contents of BNC and TNC in POC exhibited similar trends across various grassland types. Specifically, MS, DS, and SD showed a gradual decrease in BNC content with increasing soil depth, whereas TS displayed an initial increase followed by a subsequent decline (Fig. 5a and c). In contrast, FNC consistently decreased with soil depth (Fig. 5b). Within the 0–20 cm layer, the contents of BNC, FNC, and TNC in POC ranged from 0.9–4.1, 2.9–9.4, and 2.9–9.6 g kg−1, respectively. In the deeper 40–100 cm layer, these values decreased to 0.5–3.0, 1.0–4.2, and 2.1–5.9 g kg−1, respectively, with significant differences observed between the 0–20 and 40–100 cm layers (p<0.05, Fig. 5).
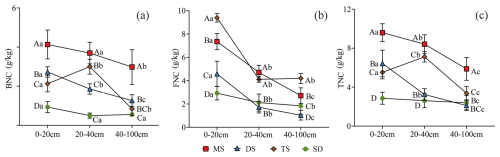
Figure 5Contents of BNC (a), FNC (b) and TNC (c) in POC in different soil layers under different grassland types. Different uppercase letters indicate significant differences in different grassland types in the same soil layer (p<0.05), and different lowercase letters indicate significant differences in different soil layers under the same grassland types (p<0.05). The red square, blue triangle, brown rhombus and green circle represent: meadow steppe (MS); desert steppe (DS); typical steppe (TS); steppe desert (SD). FNC: fungal necromass carbon, BNC: bacterial necromass carbon, TNC: total necromass carbon.
The contributions of BNC to POC were 13 %–31 %, 9 %–24 %, and 12 %–25 % in the 0–20, 20–40, and 40–100 cm layers, respectively (Fig. 6a). Similarly, FNC contributed 29 %–41 %, 19 %–38 %, and 16 %–41 % (Fig. 6b), while TNC contributed 42 %–72 %, 43 %–58 %, and 39 %–54 % to POC in the respective layers (Fig. 6c). The relationship of and were positively correlated in the 0–20 and 20–40 cm layer (R2 = 0.21, p<0.001; R2 = 0.97, p<0.0001). While, they were negative in 40–100 cm layer (R2 = 0.13, p>0.05), and no correlations at 40–100 cm layers (Fig. 6d).
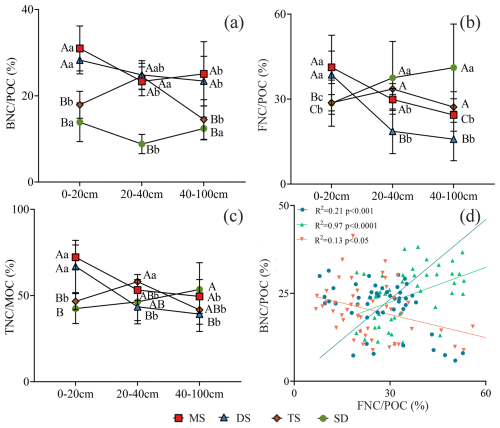
Figure 6The contribution of BNC, FNC and TNC in POC in different soil layers under different grassland types. (a) The contribution of BNC to POC; (b) the contribution of FNC to POC; (c) the contribution of TNC to POC. Different uppercase letters indicate significant differences in different grassland types in the same soil layer (p<0.05), and different lowercase letters indicate significant differences in different soil layers under the same grassland types (p<0.05). The red square, blue triangle, brown rhombus and green circle represent: meadow steppe (MS); desert steppe (DS); typical steppe (TS); steppe desert (SD). (D) the relationship between and . The blue circle, green triangle and orange inverted triangle represent the soil layers 0–20, 20–40 and 40–100 cm respectively. FNC: fungal necromass carbon, BNC: bacterial necromass carbon, POC: particulate organic carbon.
3.4 Factors affecting the contribution of MNC to MAOC and POC
Correlation analysis showed that was significantly positively correlated with TC and negatively correlated with EC and TP (p<0.05); was significantly positively correlated with MAP, Elevation, SWC, TN, AN, and SOC, and negatively correlated with pH, EC, and MAT (p<0.05); was significantly positively correlated with TC, AN, Elevation, and SOC, and negatively correlated with MAT and MAT like (p<0.05). was significantly positively correlated with Elevation, SWC, TC and SOC, and negatively correlated with MAT and EC (p<0.05); was only significantly negatively correlated with EC (p<0.05), and not significantly correlated with other influencing factors; was significantly positively correlated with Elevation, and significantly negatively correlated with MAT and EC (p<0.05). MAOC and POC were significantly negatively correlated with MAT, BD and pH, and positively correlated with MAP, Elevation, SWC, TN, AK, AN, TP and SOC in the four different types of grassland soils (p<0.05, Fig. 7).
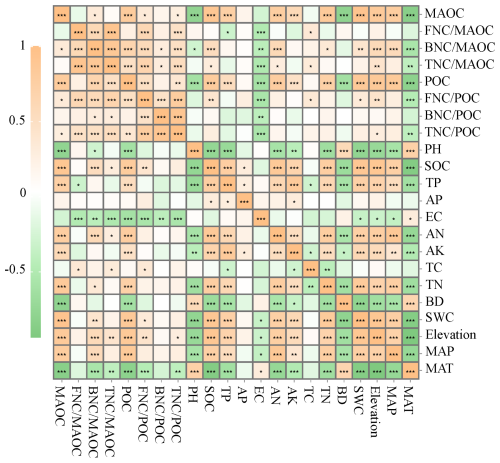
Figure 7The Spearman correlation of MAOC, POC and MNC with environmental factors in 0–100 cm soil layer under different grassland types. Colors represent Spearman correlations; MAT: mean annual temperature; MAP: mean annual precipitation; SWC: Soil water content; BD: Bulk density; TN: Total nitrogen; TC: Total carbon; TP: Total phosphorus; AK: Available potassium; AP: Available phosphorus; AN: Available nitrogen; EC: Electrical conductance; SOC: soil organic carbon. FNC : the ratio of fungal necromass carbon (bacterial necromass carbon, total necromass carbon) to mineral-associated organic carbon; FNC : the ratio of fungal necromass carbon (bacterial necromass carbon, total necromass carbon) to particulate organic carbon; * represents the Spearman correlation importance *: p<0.05; : p<0.01; : p<0.001.
3.5 Relationship between MNC in SOC fractions and soil properties
By principal component analysis, the soil properties of same soil layers in four grassland types exhibited significant variations between the 0–20 cm soil layer and the deeper 20–40 and 40–100 cm layers. In different soil layers, the contribution values of PC1 and PC2 were 44.6 % and 15.8 %, respectively. Therefore, we analyzed the soil properties affecting MNC accumulation by dividing them into 0–20 and 20–100 cm groups (Fig. S3).
The random forest model predicted the key factors affecting the accumulation of FNC, BNC, and TNC in different soil layers across various grassland types. Environmental factors such as MAP, MAT, elevation, SOC, and TN were identified as significant influencers for the accumulation of FNC, BNC, and TNC in both the 0–20 and 20–100 cm soil layers. Additionally, the influencing of EC, TP, BD and pH should not to be ignored in the 0–20 cm soil layer, while AK, AN, TP, TN, and pH also played crucial roles in the 20–100 cm soil layer.
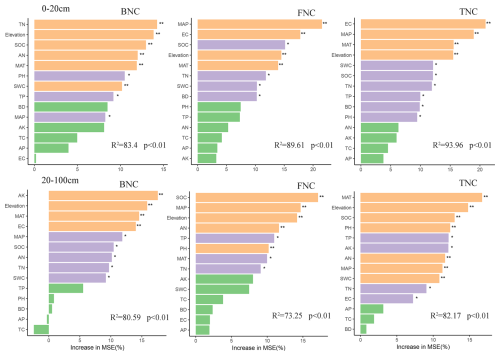
Figure 8Random forest analysis indicating the relative importance of soil properties to MNC. Colors represent significant correlations. MAT: mean annual temperature; MAP: mean annual precipitation; SWC: Soil water content; BD: Bulk density; TN: Total nitrogen; TC: Total carbon; TP: Total phosphorus; AK: Available potassium; AP: Available phosphorus; AN: Available nitrogen; EC: Electrical conductance; SOC: soil organic carbon. FNC: fungal necromass carbon, BNC: bacterial necromass carbon, TNC: total necromass carbon. * p<0.05; : p<0.01; : p<0.001 (This analysis aggregated four grassland types in same soil layer).
4.1 Distribution characteristics of SOC fractions contents in different grssland types
This study, encompassing the entire natural succession sequence in the Ningxia region, included a diverse array of plant types. Vegetation is a significant source of SOC, with the extent of root development and the composition of root exudates from diverse vegetation types exerting a direct influence on the content and distribution of SOC and its fractions (Zhao et al., 2023; Shao et al., 2021). In this study, the contents of MAOC and POC were higher than those previously reported (Zhang et al., 2024; Shen et al., 2024; Ji et al., 2020). This divergence can be attributed to variations in the input and output of organic carbon fractions, driven by differing hydrothermal conditions that affect aboveground vegetation. Additionally, researchers have noted that climate, soil, and vegetation factors significantly influence soil carbon content, with vegetation factors accounting for up to 55 % of the variation in SOC accumulation (Huang et al., 2024). The experimental period experienced increased rainfall compared to previous years, coupled with enhanced vegetation diversity and density, which collectively contributed to a greater influx of organic carbon into the soil. Consequently, this led to elevated levels of SOC and its fractions, particularly MAOC and POC.
In the 0–100 cm soil layer, the contents of MAOC and POC across different grassland types followed the order: MS > TS > DS > SD. Significant differences were observed between soil layers and grassland types (p<0.05; Fig. 1). This is because MS, compared to the other three grassland types, has higher vegetation coverage, greater root density and more abundant nutrient conditions, resulting in higher total organic carbon content and, consequently, higher soil carbon fractions (Hu et al., 2025). This study also found that the average POC content across different grassland types was higher than that of MAOC. MAOC content decreased with soil depth, this also validates our hypothesis assumption (1), while POC content in MS and TS initially increased and then decreased. Possible reasons for this include: (1) The theoretical upper limit of mineral binding with carbon in the soil, as total organic carbon increases, MAOC may reach saturation, reducing its proportion and thereby increasing the relative proportion of POC. Thus, in grasslands or soil layers with higher total organic carbon, POC content tends to be higher than MAOC (Cotrufo et al., 2019; Zhou et al., 2024, 2023). (2) Compared to DS and SD, MS and TS experience higher rainfall intensity and a more humid, colder climate (He et al., 2022; Jiang et al., 2024). Increased rainfall enhances vegetation biomass and carbon input into the soil, promoting POC formation and causing MAOC to leach into deeper layers (Chen et al., 2020c; Wang et al., 2022a). This explains the observed decrease in MAOC content with depth and the higher POC content in surface layers. (3) Increased plant biomass input has been shown to elevate POC content (Zhou et al., 2024). Given that MS and TS exhibit higher vegetation biomass per unit area compared to SD and DS, and considering that surface POC lacks physical protection, it is more susceptible to microbial decomposition (Liao et al., 2022). Therefore, in MS and TS, POC accumulation in the 20–40 cm layer is higher than in the 0–20 cm layer (p<0.05, Fig. 2). (4) Carbon inputs originate from both aboveground and belowground sources. In the 0–20 cm layer, dense surface vegetation, abundant litter, and extensive root systems contribute to increased dead root biomass, favoring POC accumulation (Liu et al., 2018). This also significantly influences soil aggregate formation and internal pore structure, enhancing POC quality (Zhang et al., 2020; Rocci et al., 2021), while having little effect on MAOC and SOC. Additionally, the desert shrub Caragana intermedia typically has a high root-to-shoot ratio (Table S1), with well-developed root systems, which also contributes to higher POC content. In deeper soil layers, where nutrient availability is significantly reduced, the rhizosphere priming effect theory suggests that microorganisms may secrete enzymes and release metabolites such as amino acids to accelerate turnover rates, leading to the utilization of MAOC and POC by microorganisms (Dijkstra et al., 2021; Cui et al., 2023). As a result, carbon fraction content in deeper layers is lower than in surface and subsurface layers, consistent with the findings of Hou et al. (2024) and Xue et al. (2023).
4.2 Contribution of MNC to different carbon fractions in different grassland types
Microbial necromass is also a key source of stable SOC pools (Min et al., 2024). The proportion of MNC in both MAOC and POC serves as a key indicator of its contribution to these carbon fractions (Hu et al., 2022). Our findings reveal that the content of TNC in MAOC and POC across different grassland types within the 0–100 cm soil layer follows a distinct order: MS > TS > DS > SD, with significant differences (p<0.05, Figs. 3 and 5). Notably, TNC content generally decreased with soil depth, except in TS, where POC-associated TNC exhibited an initial increase followed by a decline. This result partially aligns with the findings of Wang et al. (2021a), suggesting that the gradient in carbon input quantity and quality from SD to MS may enhance the efficiency of the “microbial carbon pump” (Zhu et al., 2020). Furthermore, the 0–20 cm soil layer, characterized by higher nutrient availability and more diverse microbial communities compared to deeper layers (He et al., 2024b), supports elevated microbial metabolic activity and turnover rates, thereby generating greater necromass production (Spohn et al., 2020; Li et al., 2024), This explains the observed decline in TNC within MAOC as soil depth increases. Furthermore, the ratio of FNC to BNC in same soil layers of four grassland can indirectly reflect the stability of the microbial environment (Fig. S1). A higher ratio indicates slower soil nutrient cycling and lower microbial nutrient utilization, making deeper soil layers less susceptible to external disturbances (Camenzind et al., 2021). Consequently, total organic carbon in soil fractions decreases with soil depth.
We also found that FNC content in MAOC and POC was consistently higher than BNC in the 0–100 cm soil layer (Figs. 3 and 5), and FNC's contribution to MAOC and POC was also consistently higher than BNC (Figs. 4 and 6), which is perfectly consistent with our hypothesis (2). This disparity can be attributed to the superior microbial utilization efficiency and higher carbon-to-nitrogen ratios of fungi, which facilitate more efficient necromass production compared to bacteria. Griepentrog et al. (2014) found that newly formed FNC is 2.6–4.5 times greater that of BNC. Structural and compositional differences between bacterial and fungal cell walls further influence their stability. Fungal cell walls, predominantly composed of chitin and melanin, degrade more slowly, conferring greater stability (Zhao et al., 2025), Moreover, the thicker cell walls and hyphae of fungi, coupled with their smaller surface area-to-volume ratios, promote the formation of large molecular polymers (Villarino et al., 2021), and enhance physical protection, leading to greater fungal necromass accumulation in soils. In contrast, bacteria are more likely to serve as nutrient sources under substrate-limited or nitrogen-limited conditions, rendering them more susceptible to microbial decomposition (Fernandez et al., 2019; Jia et al., 2017), which explains the lower BNC content. The importance of FNC has been emphasized in farmlands, grasslands and forests globally (Wang et al., 2021a), especially grasslands, where the chemical composition of BNC is more easily decomposed compared to FNC (Chen et al., 2021).
In summary, the average content of total MNC in MAOC and POC across different grassland types within the 0–100 cm soil layer was 4.73 and 4.96 g kg−1, respectively, with FNC content and contribution dominating. In the 0–20 cm layer, FNC and BNC contributed more significantly to MAOC, while their contributions shifted toward POC in the 20–40 and 40–100 cm layers as soil nutrient levels declined (Figs. 4 and 6). These results partially align with those of Sokol et al. (2019a, 2022). In the 0–20 cm layer, MNC primarily enters POC through “extracellular modification”, whereas in the 20–100 cm layer, it tends to integrate into the more stable MAOC via “intracellular turnover”.
4.3 Factors influencing the accumulation of MNC in different carbon fractions
Biological and abiotic factors were the primary influences on the accumulation of MNC in soil (Chen et al., 2020b; Wang et al., 2021c). In this study, key climate factors and soil properties – such as MAP, elevation, SWC, TN, TC, AK, AN, TP, and SOC – were significantly correlated with mineral-associated organic carbon (MAOC), particulate organic carbon (POC), and the ratios of and (p<0.05, Fig. 6), this result is consistent with our hypothesis (3). The relationship between MAP and SWC is particularly critical, as these factors are integral to soil nutrient cycling and energy flow (Mou et al., 2021), water availability enhances microbial growth and directly affects MNC accumulation (Hu et al., 2023). The increase in SWC can stimulate microbial activity, thereby promoting the formation and accumulation of MNC in the soil (Bell et al., 2009), On the other hand, the increase in SWC promotes plant growth and the input of organic matter into the soil, which increases the substrates available for microbial use, thus fostering microbial growth and the accumulation of MNC (Sokol et al., 2019a; Maestre et al., 2015). Compared to SD and DS, the relatively higher SWC in MS and TS to some extent explains the greater contribution of MNC in MS and TS to the organic carbon components. Higher levels of TN, TC, and TP promote microbial biomass growth, indirectly facilitating the formation of microbial necromass (Wang et al., 2021a). Moreover, BNC and FNC, as well as microbial functions, are closely related to the dynamics of C pool and N pool. The microbial demand for N can lead to the reuse of MNC by soil microbial communities, and AN can prevent the decomposition of microbial necromass (Wang et al., 2024b). As elevation increases, the decomposition rate of organic matter decreases, resulting in the accumulation of organic carbon at higher altitudes, with MNC emerging as a significant contributor to SOC. POC, being more susceptible to environmental factors, likely reflects its plant-derived origin (Soong et al., 2020). Additionally, the correlation with AP may be explained by phosphorus deficiency in soil promoting the secretion of organic acids by plant roots, which can disrupt MAOC (Ding et al., 2021). However, this conclusion requires further analysis and verification. Furthermore, MAT, EC, BD, and pH were significantly negatively correlated with MAOC, POC, and some necromass carbon fractions consistent with the findings of Wang et al. (2024a) and Zhu et al. (2024). This may be because temperature affects soil microbial respiration. He et al. (2011) found that the accumulation of MNC decreases with increasing temperature, while He et al. (2024a) concluded through meta-analysis that both aboveground and belowground plant carbon inputs increase with temperature, influencing soil microbial community structure and ultimately leading to increased MNC accumulation. Soil BD reflects changes in soil structure and aeration; lower BD improves soil permeability, enhancing microbial turnover and carbon use efficiency (Spohn et al., 2016), thereby promoting MNC accumulation. Lower pH has been shown to facilitate the accumulation of FNC and BNC (Wang et al., 2021a), consistent with the findings of Liu et al. (2024), Cui et al. (2023) and Gavazov et al. (2022). Additionally, lower pH environments are associated with higher MNC concentrations (Li et al., 2023), likely due to the ability of plant roots to acidify the soil by releasing small molecular organic acids (Fujii, 2014; Chen et al., 2020a), which in turn stimulates MNC accumulation (Wang et al., 2021b, a). Conversely, elevated pH limits MNC accumulation in SOC fractions, likely due to slower microbial turnover rates and reduced carbon use efficiency in alkaline soils (Malik et al., 2018; Tao et al., 2023). The influencing factors predicted by the random forest model were validated through correlation analysis.
In understanding the distribution and accumulation of soil carbon fractions across different grassland ecosystems, which highlight the intricate relationships between environmental variables, soil properties, and microbial necromass, offering a deeper understanding of the factors driving MNC accumulation in diverse grassland ecosystems. This study not only advances our knowledge of soil carbon dynamics but also provides a foundation for future research aimed at optimizing soil carbon sequestration strategies in response to changing environmental conditions, and have implications for soil carbon management and climate change mitigation strategies.
This study provides a comprehensive analysis of the contribution of MNC to SOC fractions and its driving factors across diverse grassland types and soil layers. In the 0–100 cm soil profile, the contents of MAOC and POC exhibited a consistent order across grassland types: MS > TS > SD > DS, with POC content generally higher than MAOC. MNC was dominated by FNC, which contributed more to MAOC and POC accumulation than BNC. A striking divergence was observed in the contribution of MNC to MAOC and POC accumulation between soil layers. In the 0–20 cm layer, FNC and BNC contributed more to MAOC accumulation than POC, while in the 20–100 cm layer, FNC and BNC contributed more to POC accumulation, showing an opposite trend between surface and deeper layers. Furthermore, the key drivers of MNC accumulation exhibited pronounced stratification across soil depths. In the 0–20 cm layer, the most influential factors on MNC accumulation were TN, MAP, and EC, while in the 20–100 cm layer, they were AK, SOC, and MAT. These findings not only elucidate the complex interplay between environmental factors and soil nitrogen-carbon dynamics but also provide a nuanced understanding of how these interactions vary with soil depth and grassland type, offering valuable implications for ecosystem management under changing environmental conditions.
The R code and datasets generated during this current study are available from the corresponding author upon reasonable request.
The supplement related to this article is available online at https://doi.org/10.5194/soil-11-883-2025-supplement.
Conceived: SC and YZ.
Writing – original draft: SC and YZ.
Review & editing: YC, JG and LC.
Analyzed the data: ML, MB, JL and JM.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors. Also, please note that this paper has not received English language copy-editing. Views expressed in the text are those of the authors and do not necessarily reflect the views of the publisher.
The authors gratefully acknowledge Profs. Jianbing Guo, and Lin Chen, and lab members, Yaqi Zhang and others, and the anonymous reviewers for their great helps and valuable suggestions.
This research has been supported by the National Natural Science Foundation of China (grant nos. 32271959, 32360423, and 32371964), the Key Research and Development Program of Ningxia (grant no. 2023BEG02049), the Ningxia Hui Autonomous Region Foreign Intelligence Introduction Programme (grant no. 2023-4), and National Key Research and Development Program of China (grant no. 2022YFF1300404).
This paper was edited by Ashish Malik and reviewed by two anonymous referees.
An, S. S., Huang, Y. M., and Liu, M. Y.: Soil Organic Carbon Density and Land Restoration: Example of Southern Mountain Area of Ningxia Province, Northwest China, Communications in Soil Science and Plant Analysis, 41, 181–189, https://doi.org/10.1080/00103620903429976, 2010.
Angst, G., Mueller, K. E., Nierop, K. G. J., and Simpson, M. J.: Plant- or microbial-derived? A review on the molecular composition of stabilized soil organic matter, Soil Biology & Biochemistry, 156, https://doi.org/10.1016/j.soilbio.2021.108189, 2021.
Bell, C. W., Acosta-Martinez, V., McIntyre, N. E., Cox, S., Tissue, D. T., and Zak, J. C.: Linking Microbial Community Structure and Function to Seasonal Differences in Soil Moisture and Temperature in a Chihuahuan Desert Grassland, Microbial Ecology, 58, 827–842, https://doi.org/10.1007/s00248-009-9529-5, 2009.
Bölscher, T., Vogel, C., Olagoke, F. K., Meurer, K. H. E., Herrmann, A. M., Colombi, T., Brunn, M., and Domeignoz-Horta, L. A.: Beyond growth: The significance of non-growth anabolism for microbial carbon-use efficiency in the light of soil carbon stabilisation, Soil Biology & Biochemistry, 193, https://doi.org/10.1016/j.soilbio.2024.109400, 2024.
Camenzind, T., Grenz, K. P., Lehmann, J., and Rillig, M. C.: Soil fungal mycelia have unexpectedly flexible stoichiometric C:N and C:P ratios, Ecology Letters, 24, 208–218, https://doi.org/10.1111/ele.13632, 2021.
Chai, J., Ling, Z. B., Wang, Y., Dong, R., Zheng, Y. H., and Qi, J. T.: A method for measuring soil water content based on principal component analysis, Review of Scientific Instruments, 95, https://doi.org/10.1063/5.0178324, 2024.
Chen, C., Li, Z. B., Li, S. J., Deng, N. X., and Mei, P.: Effects of root exudates on the activation and remediation of cadmium ion in contaminated soils, Environmental Science and Pollution Research, 27, 2926–2934, https://doi.org/10.1007/s11356-019-07263-8, 2020a.
Chen, G. P., Ma, S. H., Tian, D., Xiao, W., Jiang, L., Xing, A. J., Zou, A. L., Zhou, L. H., Shen, H. H., Zheng, C. Y., Ji, C. J., He, H. B., Zhu, B., Liu, L. L., and Fang, J. Y.: Patterns and determinants of soil microbial residues from tropical to boreal forests, Soil Biology & Biochemistry, 151, https://doi.org/10.1016/j.soilbio.2020.108059, 2020b.
Chen, J. G., Xiao, W., Zheng, C. Y., and Zhu, B.: Nitrogen addition has contrasting effects on particulate and mineral-associated soil organic carbon in a subtropical forest, Soil Biology & Biochemistry, 142, https://doi.org/10.1016/j.soilbio.2020.107708, 2020c.
Chen, X. B., Hu, Y. J., Xia, Y. H., Zheng, S. M., Ma, C., Rui, Y. C., He, H. B., Huang, D. Y., Zhang, Z. H., Ge, T. D., Wu, J. S., Guggenberger, G., Kuzyakov, Y., and Su, Y. R.: Contrasting pathways of carbon sequestration in paddy and upland soils, Global Change Biology, 27, 2478–2490, https://doi.org/10.1111/gcb.15595, 2021.
Cotrufo, M. F., Ranalli, M. G., Haddix, M. L., Six, J., and Lugato, E.: Soil carbon storage informed by particulate and mineral-associated organic matter, Nature Geoscience, 12, https://doi.org/10.1038/s41561-019-0484-6, 2019.
Cui, H., Mo, C. Y., Chen, P. F., Lan, R., He, C., Lin, J. D., Jiang, Z. H., and Yang, J. P.: Impact of rhizosphere priming on soil organic carbon dynamics: Insights from the perspective of carbon fractions, Applied Soil Ecology, 189, https://doi.org/10.1016/j.apsoil.2023.104982, 2023.
Deng, F. B. and Liang, C.: Revisiting the quantitative contribution of microbial necromass to soil carbon pool: Stoichiometric control by microbes and soil, Soil Biology & Biochemistry, 165, https://doi.org/10.1016/j.soilbio.2021.108486, 2022.
Dijkstra, F. A., Zhu, B., and Cheng, W. X.: Root effects on soil organic carbon: a double-edged sword, New Phytologist, 230, 60–65, https://doi.org/10.1111/nph.17082, 2021.
Ding, W. L., Cong, W. F., and Lambers, H.: Plant phosphorus-acquisition and -use strategies affect soil carbon cycling, Trends in Ecology & Evolution, 36, 899–906, https://doi.org/10.1016/j.tree.2021.06.005, 2021.
Ding, X. L., Chen, S. Y., Zhang, B., Liang, C., He, H. B., and Horwath, W. R.: Warming increases microbial residue contribution to soil organic carbon in an alpine meadow, Soil Biology & Biochemistry, 135, 13–19, https://doi.org/10.1016/j.soilbio.2019.04.004, 2019.
Du, L. T., Gong, F., Zeng, Y. J., Ma, L. L., Qiao, C. L., and Wu, H. Y.: Carbon use efficiency of terrestrial ecosystems in desert/grassland biome transition zone: A case in Ningxia province, northwest China, Ecological Indicators, 120, https://doi.org/10.1016/j.ecolind.2020.106971, 2021.
Dou, M. K., Zhang, W. D., Yang, Q. P., Chen, L. C., Liu, Y. J., and Hu, Y. L.: Effects of Chinese fir planting and phosphorus addition on soil microbial biomass, Chinese Journal of Applied Ecology, 34, 631–638, https://doi.org/10.13287/j.1001-9332.202303.003, 2023.
Fernandez, C. W., Heckman, K., Kolka, R., and Kennedy, P. G.: Melanin mitigates the accelerated decay of mycorrhizal necromass with peatland warming, Ecology Letters, 22, 498–505, https://doi.org/10.1111/ele.13209, 2019.
Fujii, K.: Soil acidification and adaptations of plants and microorganisms in Bornean tropical forests, Ecological Research, 29, 371–381, https://doi.org/10.1007/s11284-014-1144-3, 2014.
Gavazov, K., Canarini, A., Jassey, V. E. J., Mills, R., Richter, A., Sundqvist, M. K., Väisänen, M., Walker, T. W. N., Wardle, D. A., and Dorrepaal, E.: Plant-microbial linkages underpin carbon sequestration in contrasting mountain tundra vegetation types, Soil Biology & Biochemistry, 165, https://doi.org/10.1016/j.soilbio.2021.108530, 2022.
Griepentrog, M., Bodé, S., Boeckx, P., Hagedorn, F., Heim, A., and Schmidt, M. W. I.: Nitrogen deposition promotes the production of new fungal residues but retards the decomposition of old residues in forest soil fractions, Global Change Biology, 20, 327–340, https://doi.org/10.1111/gcb.12374, 2014.
Hao, Z. G., Zhao, Y. F., Wang, X., Wu, J. H., Jiang, S. L., Xiao, J. N., Wang, K. C., Zhou, X. H., Liu, H. Y., Li, J., and Sun, Y. X.: Thresholds in aridity and soil carbon-to-nitrogen ratio govern the accumulation of soil microbial residues, Communications Earth & Environment, 2, https://doi.org/10.1038/s43247-021-00306-4, 2021.
He, H. B., Zhang, W., Zhang, X. D., Xie, H. T., and Zhuang, J.: Temporal responses of soil microorganisms to substrate addition as indicated by amino sugar differentiation, Soil Biology & Biochemistry, 43, 1155–1161, https://doi.org/10.1016/j.soilbio.2011.02.002, 2011.
He, J. H., Nie, Y. X., Tan, X. P., Hu, A., Li, Z. Q., Dai, S. P., Ye, Q., Zhang, G. X., and Shen, W. J.: Latitudinal patterns and drivers of plant lignin and microbial necromass accumulation in forest soils: Disentangling microbial and abiotic controls, Soil Biology & Biochemistry, 194, https://doi.org/10.1016/j.soilbio.2024.109438, 2024a.
He, L. B., Sun, X. Y., Li, S. Y., Zhou, W. Z., Yu, J. T., Zhao, G. Y., Chen, Z., Bai, X. T., and Zhang, J. S.: Depth effects on bacterial community altitudinal patterns and assembly processes in the warm-temperate montane forests of China, Science of the Total Environment, 914, https://doi.org/10.1016/j.scitotenv.2024.169905, 2024b.
He, M., Fang, K., Chen, L. Y., Feng, X. H., Qin, S. Q., Kou, D., He, H. B., Liang, C., and Yang, Y. H.: Depth-dependent drivers of soil microbial necromass carbon across Tibetan alpine grasslands, Global Change Biology, 28, 936–949, https://doi.org/10.1111/gcb.15969, 2022.
Hou, Z. N., Wang, R. H., Chang, S., Zheng, Y., Ma, T. T., Xu, S. Q., Zhang, X. J., Shi, X., Lu, J., Luo, D. Q., Wang, B., Du, Z. L., and Wei, Y. Q.: The contribution of microbial necromass to soil organic carbon and influencing factors along a variation of habitats in alpine ecosystems, Science of the Total Environment, 921, https://doi.org/10.1016/j.scitotenv.2024.171126, 2024.
Hu, J. X., Du, M. L., Chen, J., Tie, L. H., Zhou, S. X., Buckeridge, K. M., Cornelissen, J. H. C., Huang, C. D., and Kuzyakov, Y.: Microbial necromass under global change and implications for soil organic matter, Global Change Biology, 29, 3503–3515, https://doi.org/10.1111/gcb.16676, 2023.
Hu, P. L., Zhang, W., Chen, H. S., Xu, L., Xiao, J., Luo, Y. Q., and Wang, K. L.: Lithologic control of microbial-derived carbon in forest soils, Soil Biology & Biochemistry, 167, https://doi.org/10.1016/j.soilbio.2022.108600, 2022.
Hu, Y., Fu, L., Ao, G., Ji, C., Zeng, H., and Zhu, B.: Climate, plant and microorganisms jointly influence soil organic matter fractions in temperate grasslands, The Science of the Total Environment, 958, 178133, https://doi.org/10.1016/j.scitotenv.2024.178133, 2025.
Hua, H., Qian, C., Xue, K., Jörgensen, R. G., Keiluweit, M., Liang, C., Zhu, X. F., Chen, J., Sun, Y. S., Ni, H. W., Ding, J. X., Huang, W. G., Mao, J. D., Tan, R. X., Zhou, J. Z., Crowther, T. W., Zhou, Z. H., Zhang, J. B., and Liang, Y. T.: Reducing the uncertainty in estimating soil microbial- derived carbon storage, Proceedings of the National Academy of Sciences of the United States of America, 121, https://doi.org/10.1073/pnas.2401916121, 2024.
Huang, L. X., Gao, Y., Wang, D. F., Cui, X. J., Zhang, H. M., Yuan, J. M., and Gao, M. M.: Natural grassland restoration exhibits enhanced carbon sequestration and soil improvement potential in northern sandy grasslands of China: An empirical study, Catena, 246, https://doi.org/10.1016/j.catena.2024.108396, 2024.
Indorf, C., Dyckmans, J., Khan, K. S., and Joergensen, R. G.: Optimisation of amino sugar quantification by HPLC in soil and plant hydrolysates, Biology and Fertility of Soils, 47, 387–396, https://doi.org/10.1007/s00374-011-0545-5, 2011.
Ji, B., Xie, Y.-Z., He, J.-L., Wang, Z.-J., and Jiang, Q.: Carbon sequestration characteristics of typical temperate natural grasslands in Ningxia, China, The Journal of Applied Ecology, 31, 3657–3664, https://doi.org/10.13287/j.1001-9332.202011.010, 2020.
Ji, X. L., Wu, D., Yan, Y. G., Guo, W., and Li, K.: Interpreting regional ecological security from perspective of ecological networks: a case study in Ningxia Hui Autonomous Region, China, Environmental Science and Pollution Research, 30, 65412–65426, https://doi.org/10.1007/s11356-023-26997-0, 2023.
Jia, J., Feng, X. J., He, J. S., He, H. B., Lin, L., and Liu, Z. G.: Comparing microbial carbon sequestration and priming in the subsoil versus topsoil of a Qinghai-Tibetan alpine grassland, Soil Biology & Biochemistry, 104, 141–151, https://doi.org/10.1016/j.soilbio.2016.10.018, 2017.
Jiang, M. D., Li, H. L., Zhang, W., Liu, J. B., and Zhang, Q.: Effects of climate change and grazing on the soil organic carbon stock of alpine wetlands on the Tibetan Plateau from 2000 to 2018, Catena, 238, https://doi.org/10.1016/j.catena.2024.107870, 2024.
Lehmann, J. and Kleber, M.: The contentious nature of soil organic matter, Nature, 528, 60–68, https://doi.org/10.1038/nature16069, 2015.
Li, D. J., Liu, J., Chen, H., Zheng, L., and Wang, K. L.: Soil gross nitrogen transformations in responses to land use conversion in a subtropical karst region, Journal of Environmental Management, 212, 1–7, https://doi.org/10.1016/j.jenvman.2018.01.084, 2018.
Li, J., Zhang, Q., Li, Y., Liu, Y., Xu, J., and Di, H.: Effects of long-term mowing on the fractions and chemical composition of soil organic matter in a semiarid grassland, Biogeosciences, 14, 2685–2696, https://doi.org/10.5194/bg-14-2685-2017, 2017.
Li, N., Zhao, N., Xu, S. X., Wang, Y. L., Wei, L., Zhang, Q., Guo, T. Q., and Wang, X. A.: Accumulation of microbial necromass carbon and its contribution to soil organic carbon in artificial grasslands of various vegetation types, European Journal of Soil Biology, 119, https://doi.org/10.1016/j.ejsobi.2023.103573, 2023.
Li, Y., Wang, B. R., Zhang, Y. H., Ao, D., Feng, C. L., Wang, P., Bai, X. J., and An, S. S.: Afforestation increased the microbial necromass carbon accumulation in deep soil on the Loess Plateau, Journal of Environmental Management, 349, https://doi.org/10.1016/j.jenvman.2023.119508, 2024.
Liang, C., Amelung, W., Lehmann, J., and Kästner, M.: Quantitative assessment of microbial necromass contribution to soil organic matter, Global Change Biology, 25, 3578–3590, https://doi.org/10.1111/gcb.14781, 2019.
Liao, C., Men, X., Wang, C., Chen, R., and Cheng, X. L.: Nitrogen availability and mineral particles contributed fungal necromass to the newly formed stable carbon pool in the alpine areas of Southwest China, Soil Biology & Biochemistry, 173, https://doi.org/10.1016/j.soilbio.2022.108788, 2022.
Liao, J. J., Yang, X., Dou, Y. X., Wang, B. R., Xue, Z. J., Sun, H., Yang, Y., and An, S. S.: Divergent contribution of particulate and mineral-associated organic matter to soil carbon in grassland, Journal of Environmental Management, 344, https://doi.org/10.1016/j.jenvman.2023.118536, 2023.
Liu, H. Y., Mi, Z. R., Lin, L., Wang, Y. H., Zhang, Z. H., Zhang, F. W., Wang, H., Liu, L. L., Zhu, B. A., Cao, G. M., Zhao, X. Q., Sanders, N. J., Classen, A. T., Reich, P. B., and He, J. S.: Shifting plant species composition in response to climate change stabilizes grassland primary production, Proceedings of the National Academy of Sciences of the United States of America, 115, 4051–4056, https://doi.org/10.1073/pnas.1700299114, 2018.
Liu, X. F., Tian, Y., Heinzle, J., Salas, E., Kwatcho-Kengdo, S., Borken, W., Schindlbacher, A., and Wanek, W.: Long-term soil warming decreases soil microbial necromass carbon by adversely affecting its production and decomposition, Global Change Biology, 30, https://doi.org/10.1111/gcb.17379, 2024.
Maestre, F. T., Delgado-Baquerizo, M., Jeffries, T. C., Eldridge, D. J., Ochoa, V., Gozalo, B., Quero, J. L., García-Gómez, M., Gallardo, A., Ulrich, W., Bowker, M. A., Arredondo, T., Barraza-Zepeda, C., Bran, D., Florentino, A., Gaitán, J., Gutiérrez, J. R., Huber-Sannwald, E., Jankju, M., Mau, R. L., Miriti, M., Naseri, K., Ospina, A., Stavi, I., Wang, D. L., Woods, N. N., Yuan, X., Zaady, E., and Singh, B. K.: Increasing aridity reduces soil microbial diversity and abundance in global drylands, Proceedings of the National Academy of Sciences of the United States of America, 112, 15684–15689, https://doi.org/10.1073/pnas.1516684112, 2015.
Malik, A. A., Puissant, J., Buckeridge, K. M., Goodall, T., Jehmlich, N., Chowdhury, S., Gweon, H. S., Peyton, J. M., Mason, K. E., van Agtmaal, M., Blaud, A., Clark, I. M., Whitaker, J., Pywell, R. F., Ostle, N., Gleixner, G., and Griffiths, R. I.: Land use driven change in soil pH affects microbial carbon cycling processes, Nat. Commun., 9, https://doi.org/10.1038/s41467-018-05980-1, 2018.
Min, K. K., Lynch, L., Zheng, T. T., Chen, F. S., and Liang, C.: Factors driving microbial biomass and necromass relationships display ecosystem-dependent responses, European Journal of Soil Science, 75, https://doi.org/10.1111/ejss.13555, 2024.
Mou, Z. J., Kuang, L. H., He, L. F., Zhang, J., Zhang, X. Y., Hui, D. F., Li, Y., Wu, W. J., Mei, Q. M., He, X. J., Kuang, Y. W., Wang, J., Wang, Y. Q., Lambers, H., Sardans, J., Peñuelas, J., and Liu, Z. F.: Climatic and edaphic controls over the elevational pattern of microbial necromass in subtropical forests, Catena, 207, https://doi.org/10.1016/j.catena.2021.105707, 2021.
Roberts, P., Bol, R., and Jones, D. L.: Free amino sugar reactions in soil in relation to soil carbon and nitrogen cycling, Soil Biology & Biochemistry, 39, 3081–3092, https://doi.org/10.1016/j.soilbio.2007.07.001, 2007.
Rocci, K. S., Lavallee, J. M., Stewart, C. E., and Cotrufo, M. F.: Soil organic carbon response to global environmental change depends on its distribution between mineral-associated and particulate organic matter: A meta-analysis, Science of the Total Environment, 793, https://doi.org/10.1016/j.scitotenv.2021.148569, 2021.
Shao, P. S., Lynch, L., Xie, H. T., Bao, X. L., and Liang, C.: Tradeoffs among microbial life history strategies influence the fate of microbial residues in subtropical forest soils, Soil Biology & Biochemistry, 153, https://doi.org/10.1016/j.soilbio.2020.108112, 2021.
Shen, A. H., Shi, Y., Mi, W. B., Yue, S. L., She, J., Zhang, F. H., Guo, R., He, H. Y., Wu, T., Li, H. X., and Zhao, N.: Effects of desert plant communities on soil enzyme activities and soil organic carbon in the proluvial fan in the eastern foothills of the Helan Mountain in Ningxia, China, Journal of Arid Land, 16, 725–737, https://doi.org/10.1007/s40333-024-0076-1, 2024.
Sokol, N. W., Sanderman, J., and Bradford, M. A.: Pathways of mineral-associated soil organic matter formation: Integrating the role of plant carbon source, chemistry, and point of entry, Global Change Biology, 25, 12–24, https://doi.org/10.1111/gcb.14482, 2019a.
Sokol, N. W., Kuebbing, S. E., Karlsen-Ayala, E., and Bradford, M. A.: Evidence for the primacy of living root inputs, not root or shoot litter, in forming soil organic carbon, New Phytologist, 221, 233–246, https://doi.org/10.1111/nph.15361, 2019b.
Sokol, N. W., Slessarev, E., Marschmann, G. L., Nicolas, A., Blazewicz, S. J., Brodie, E. L., Firestone, M. K., Foley, M. M., Hestrin, R., Hungate, B. A., Koch, B. J., Stone, B. W., Sullivan, M. B., Zablocki, O., Pett-Ridge, J., and Consortium, L. S. M.: Life and death in the soil microbiome: how ecological processes influence biogeochemistry, Nature Reviews Microbiology, 20, 415–430, https://doi.org/10.1038/s41579-022-00695-z, 2022.
Soong, J. L., Fuchslueger, L., Marañon-Jimenez, S., Torn, M. S., Janssens, I. A., Penuelas, J., and Richter, A.: Microbial carbon limitation: The need for integrating microorganisms into our understanding of ecosystem carbon cycling, Global Change Biology, 26, 1953–1961, https://doi.org/10.1111/gcb.14962, 2020.
Spohn, M., Klaus, K., Wanek, W., and Richter, A.: Microbial carbon use efficiency and biomass turnover times depending on soil depth – Implications for carbon cycling, Soil Biology & Biochemistry, 96, 74–81, https://doi.org/10.1016/j.soilbio.2016.01.016, 2016.
Spohn, M., Müller, K., Höschen, C., Mueller, C. W., and Marhan, S.: Dark microbial CO2 fixation in temperate forest soils increases with CO2 concentration, Global Change Biology, 26, 1926-1935, https://doi.org/10.1111/gcb.14937, 2020.
Tao, F., Huang, Y. Y., Hungate, B. A., Manzoni, S., Frey, S. D., Schmidt, M. W. I., Reichstein, M., Carvalhais, N., Ciais, P., Jiang, L. F., Lehmann, J., Wang, Y. P., Houlton, B. Z., Ahrens, B., Mishra, U., Hugelius, G., Hocking, T. D., Lu, X. J., Shi, Z., Viatkin, K., Vargas, R., Yigini, Y., Omuto, C., Malik, A. A., Peralta, G., Cuevas-Corona, R., Di Paolo, L. E., Luotto, I., Liao, C. J., Liang, Y. S., Saynes, V. S., Huang, X. M., and Luo, Y. Q.: Microbial carbon use efficiency promotes global soil carbon storage, Nature, 618, https://doi.org/10.1038/s41586-023-06042-3, 2023.
Villarino, S. H., Pinto, P., Jackson, R. B., and Piñeiro, G.: Plant rhizodeposition: A key factor for soil organic matter formation in stable fractions, Science Advances, 7, https://doi.org/10.1126/sciadv.abd3176, 2021.
Wang, B. R., An, S. S., Liang, C., Liu, Y., and Kuzyakov, Y.: Microbial necromass as the source of soil organic carbon in global ecosystems, Soil Biology & Biochemistry, 162, https://doi.org/10.1016/j.soilbio.2021.108422, 2021a.
Wang, B. R., Liang, C., Yao, H. J., Yang, E., and An, S. S.: The accumulation of microbial necromass carbon from litter to mineral soil and its contribution to soil organic carbon sequestration, Catena, 207, https://doi.org/10.1016/j.catena.2021.105622, 2021b.
Wang, B. R., Huang, Y. M., Li, N., Yao, H. J., Yang, E., Soromotin, A., Kuzyakov, Y., Cheptsov, V., Yang, Y., and An, S. S.: Initial soil formation by biocrusts: Nitrogen demand and clay protection control microbial necromass accrual and recycling, Soil Biology & Biochemistry, 167, https://doi.org/10.1016/j.soilbio.2022.108607, 2022a.
Wang, C., Qu, L. R., Yang, L. M., Liu, D. W., Morrissey, E., Miao, R. H., Liu, Z. P., Wang, Q. K., Fang, Y. T., and Bai, E.: Large-scale importance of microbial carbon use efficiency and necromass to soil organic carbon, Global Change Biology, 27, 2039–2048, https://doi.org/10.1111/gcb.15550, 2021c.
Wang, S. C., Wang, Z. Q., Heinonsalo, J., Zhang, Y. X., and Liu, G.: Soil organic carbon stocks and dynamics in a mollisol region: A 1980s–2010s study, Science of the Total Environment, 807, https://doi.org/10.1016/j.scitotenv.2021.150910, 2022b.
Wang, X., Liang, C., Dini-Andreote, F., Zhou, S., and Jiang, Y.: Impacts of trophic interactions on carbon accrual in soils, Trends in Microbiology, https://doi.org/10.1016/j.tim.2024.10.009, 2024a.
Wang, X. X., Zhou, L. Y., Fu, Y. L., Jiang, Z., Jia, S. X., Song, B. Q., Liu, D. Q., and Zhou, X. H.: Drought-induced changes in rare microbial community promoted contribution of microbial necromass C to SOC in a subtropical forest, Soil Biology & Biochemistry, 189, https://doi.org/10.1016/j.soilbio.2023.109252, 2024b.
Wu, M. Y., Chen, L., Chen, S. G., Chen, Y. L., Ma, J. P., Zhang, Y. Q., Pang, D. B., and Li, X. B.: Soil microbial carbon and nitrogen limitation constraints soil organic carbon stability in arid and semi-arid grasslands, Journal of Environmental Management, 373, https://doi.org/10.1016/j.jenvman.2024.123675, 2025.
Xue, Z.-J., Qu, T.-T., Liu, C.-H., Liu, X.-K., Wang, R., Wang, N., Zhou, Z.-C., and Dong, Z.-B.: Contribution of microbial necromass to soil organic carbon formation during litter decomposition under incubation conditions, The Journal of Applied Ecology, 34, 1845–1852, https://doi.org/10.13287/j.1001-9332.202307.004, 2023.
Yang, Y., Wang, B.-R., Dou, Y.-X., Xue, Z.-J., Sun, H., Wang, Y.-Q., Liang, C., and An, S.-S.: Advances in the research of transformation and stabilization of soil organic carbon from plant and microbe, The Journal of Applied Ecology, 35, 111–123, https://doi.org/10.13287/j.1001-9332.202401.011, 2024.
Zhang, X.-F., Zheng, S.-M., Xia, Y.-H., Hu, Y.-J., Su, Y.-R., and Chen, X.-B.: Responses of Soil Organic Carbon Fractions to Land Use Types in Hilly Red Soil Regions, China, Huan jing ke xue = Huanjing kexue, 41, 1466–1473, https://doi.org/10.13227/j.hjkx.201908218, 2020.
Zhang, X. J., Wang, D. N., Ma, K. X., Sun, D., Yang, F. L., and Lin, H. L.: Spatiotemporal evolution of soil water erosion in Ningxia grassland based on the RUSLE-TLSD model, Environmental Research, 236, https://doi.org/10.1016/j.envres.2023.116744, 2023.
Zhang, Y., Cheng, C. X., Wang, Z. H., Hai, H. X., and Miao, L. L.: Spatiotemporal Variation and Driving Factors of Carbon Sequestration Rate in Terrestrial Ecosystems of Ningxia, China, Land, 14, https://doi.org/10.3390/land14010094, 2025.
Zhang, Y.-H., Li, Y., Zhou, Y., Chen, Y.-J., and An, S.-S.: Changes of soil nutrients and organic carbon fractions in Caragana korshinskii forests with different restoration years in mountainous areas of southern Ningxia, China, The Journal of Applied Ecology, 35, 639–647, https://doi.org/10.13287/j.1001-9332.202403.018, 2024.
Zhang, Z. F., Pan, Y. P., Liu, Y., and Li, M.: High-Level Diversity of Basal Fungal Lineages and the Control of Fungal Community Assembly by Stochastic Processes in Mangrove Sediments, Applied and Environmental Microbiology, 87, https://doi.org/10.1128/aem.00928-21, 2021.
Zhao, Q. Z., Shi, P., Li, P., Li, Z. B., Min, Z. Q., Sun, J. M., Cui, L. Z., Niu, H. B., Zu, P. J., and Cao, M. H.: Effects of vegetation restoration on soil organic carbon in the Loess Plateau: A meta-analysis, Land Degradation & Development, 34, 2088–2097, https://doi.org/10.1002/ldr.4591, 2023.
Zhao, Y. D., Li, D. S., and Zhou, J. X.: Microbial necromass as a critical driver of soil organic carbon accumulation in Qinghai-Tibet Plateau under climate warming: A meta-analysis, Geoderma Regional, 40, https://doi.org/10.1016/j.geodrs.2024.e00903, 2025.
Zhou, H., Yan, Y. J., Dai, Q. H., He, Z. J., and Yi, X. S.: Latitudinal and Altitudinal Patterns and Influencing Factors of Soil Humus Carbon in the Low-Latitude Plateau Regions, Forests, 14, https://doi.org/10.3390/f14020344, 2023.
Zhou, Y., Li, Y.-Y., Li, N., Li, H.-J., Zhang, Y.-H., An, S.-S., and Wang, B.-R.: Contribution of soil microbial necromass carbon to soil organic carbon in grassland under precipitation change and its influencing factors in loess hilly region, Northwest China, The Journal of Applied Ecology, 35, 2592–2598, https://doi.org/10.13287/j.1001-9332.202409.011, 2024.
Zhu, X. F., Jackson, R. D., DeLucia, E. H., Tiedje, J. M., and Liang, C.: The soil microbial carbon pump: From conceptual insights to empirical assessments, Global Change Biology, 26, 6032–6039, https://doi.org/10.1111/gcb.15319, 2020.
Zhu, X. F., Min, K. K., Feng, K., Xie, H. T., He, H. B., Zhang, X. D., Deng, Y., and Liang, C.: Microbial necromass contribution to soil carbon storage via community assembly processes, Science of the Total Environment, 951, https://doi.org/10.1016/j.scitotenv.2024.175749, 2024.





